 U.S. Senators Dick Durbin (D-IL) and Richard Blumenthal (D-CT) are calling on the FDA to investigate manufacturers of dietary supplements who list “acacia rigidula” as an ingredient in their products. That phrase masks the addition of BMPEA, a dangerous synthetic amphetamine.
U.S. Senators Dick Durbin (D-IL) and Richard Blumenthal (D-CT) are calling on the FDA to investigate manufacturers of dietary supplements who list “acacia rigidula” as an ingredient in their products. That phrase masks the addition of BMPEA, a dangerous synthetic amphetamine.
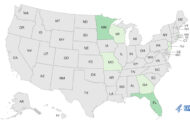
A study released earlier this month in Analytical Chemistry found the drug in 11 out of 21 of over-the-counter dietary supplements. The supplements that contain BMPEA, or β-methylphenylethylamine, increased from 42.9% in 2012 to 52.4% in 2014.
The Senators wrote, “for too long, dietary supplement manufacturers have either failed to list BMPEA on product labels or have listed the stimulant as a ‘natural botanical’ which the Food and Drug Administration’s own scientists have disproved. Other countries and entities have already taken note of A. rigidula and BMPEA’s potential danger. We are very troubled by FDA’s inaction on this issue.”
The drug is classified as a doping agent by the World Anti-Doping Agency. It has never been tested for efficacy or safety on human beings, although tests on animals has shown it can dangerously increase blood pressure. As a class of drugs, amphetamines cause increased blood pressure, heart rate, and body temperature, which can lead to strokes at high doses, suppressed sleep and appetite. Amphetamines are also addictive.
In addition, according to the letter sent to the FDA, “FDA scientists concluded that it is nearly impossible for the amounts of BMPEA present in dietary supplements to be derived from A. rigidula plant extracts.” that means that the drug is deliberately added to the supplements in violation of the Dietary Supplement Health and Education Act of 1994.