The Centers for Disease Control and Prevention have updated their investigation into the fungal meningitis outbreak that has sickened people who had epidural steroid injections from drugs made by the New England Compounding Center (NECC) for back trouble and sciatica. Now 170 people are sick in 11 states, and 14 people have died.
Attorney Fred Pritzker has filed and won many lawsuits in product liability cases. He has been contacted by some of the patients in this outbreak and has called on the officers of the New England Compounding Pharmacy, doing business as the NECC, to answer questions about its business practices. Pritzker said, “the company needs to take responsibility for these injuries and disclose information that will prevent further harm. We also need to know how the drugs were produced and how the contamination occurred.”
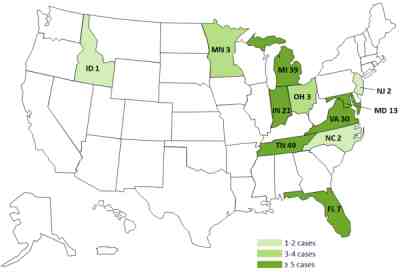
The case count by states is as follows: Florida (7, with 2 deaths), Idaho (1), Indiana (21, with 1 death), Maryland (13, with 1 death), Michigan (39, with 3 deaths), Minnesota (3), New Jersey (3), North Carolina (2), Ohio (3), Tennessee (49, with 6 deaths), and Virginia (30, with 1 death). The presence of the fungus Exserohilum has been found in 10 patients, and the fungus Aspergillus in one person.
Because the onset of symptoms occurs 1 to 4 weeks after injection, the case count is expected to increase. The periods of time between injection and onset of symptoms has varied, with some shorter and some longer. The injections were given starting May 21, 2012.
The FDA expanded the recall of the potentially contaminated drugs on October 6, 2012. Clinics and pain centers that received the drugs have been notified and instructed to notify their patients about this problem. The only people at risk in this outbreak are those who received injections of methylprednisolone acetate from NECC between May 21 and September 28, 2012. This type of meningitis is not contagious.




