Invisiblu International LLC is voluntarily recalling one lot of Continuum Labs LGD-Xtreme because it contains LGD-4033 Ligandrol. The risks of this product are unknown. There have been no reports of adverse events related to the consumption of this product.
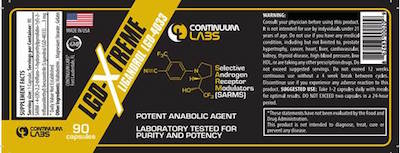 LGD-Xtreme is marketed as a dietary supplement to promote gains of lean muscle mass. The recalled product is in 3 mg tablets. The product is packaged in a dark amber plastic bottle with ninety capsules. The lot numbers are 21511166 with an expiration date of 11/2018. The product is identified by its black label with gold trim and the Continuum Labs logo. It was sold to consumers in the United States over the internet, and was exported to wholesalers in Brazil.
LGD-Xtreme is marketed as a dietary supplement to promote gains of lean muscle mass. The recalled product is in 3 mg tablets. The product is packaged in a dark amber plastic bottle with ninety capsules. The lot numbers are 21511166 with an expiration date of 11/2018. The product is identified by its black label with gold trim and the Continuum Labs logo. It was sold to consumers in the United States over the internet, and was exported to wholesalers in Brazil.
If you purchased this product, stop using it immediately and throw away any unused capsules. If you have been experiencing any problems that may be related to this drug product, see your doctor immediately. Any adverse reactions may be reported to the FDA’s MedWatch Adverse Event Reporting program.




