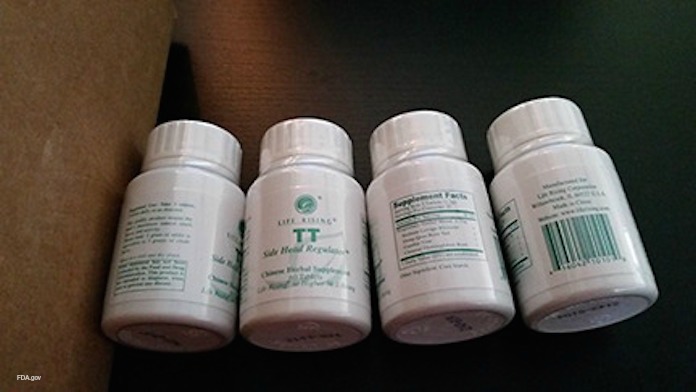
Ton Shen Health of Chicago is recalling its Life Rising brand of “Side Head Regulator TT” Tablets because they are positive for elevated levels of lead for children under the age of 18. This is the same firm that recalled dietary supplements DHZC-2 Tablets for lead in August 2016, which were linked to the illnesses of two children and the possibility of two deaths. No illnesses have been reported to date in connection with this newly recalled product.
The recalled product is Life Rising brand “Side Head Regulator TT” tablets that were sold mostly locally in Chicago area retail stores. Some were distributed to other states via mail order. The product is in a 1.6 ounce, white plastic package marked with the lot number on the bottom. The UPC number is 616042101010.
Lead poisoning can occur if a person is exposed to high levels of lead over short periods of time. High lead levels can cause damage to the nervous system and internal organs, but people may have no symptoms for long term poisoning. In short term poisoning, symptoms include abdominal pain, muscle weakness, nausea, vomiting, weight loss, and bloody or decreased urine output. Children are especially vulnerable to lead poisoning. If your child consumed this product, talk to your health care provider about testing for lead.
The sale of the product has been suspended by Ton Shen Health at the end of September 2016. If you purchased this product, do not consume it. Throw it away or take it back to the place of purchase for a refund.