 Enhanced laboratory detection of non-O157:H7 types of E. coli from stool samples of the sick is causing a surge in positive findings for toxic E. coli that is exponentially larger than the number of labs adopting the enhanced methods, a study by a Washington State Department of Health has found.
Enhanced laboratory detection of non-O157:H7 types of E. coli from stool samples of the sick is causing a surge in positive findings for toxic E. coli that is exponentially larger than the number of labs adopting the enhanced methods, a study by a Washington State Department of Health has found.
Public health laboratories have long been focused on detecting E. coli O157:H7, a virulent bacteria that was first found in the 1970s to be causing outbreaks of bloody diarrhea and life-threatening hemolytic uremic syndrome (HUS) traced to under-cooked ground beef.
Only in the past decade has there been a broader realization that other types of Shiga toxin-producing E. coli (STECs) are just as dangerous. Later this year under a federal rule change, meatpackers will be required to expand sample testing to detect six types of non-O157:H7 STEC.
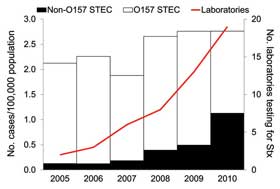
Washington State disease trackers looked at laboratories in Washington and examined the rate of non-O157 E. coli detection as more and more labs developed the tools to test for varied STECs. According to a summary of the research published this week by the Centers for Disease Control and Prevention (CDC), the number of laboratories in Washington State that tested for varied STECs increased from 2 (4 percent) in 2005 to 19 (33 percent) in 2010.
Over that period, the incidence of reported non-O157 STEC infections increased from 8 cases to 76 cases in 2010. The most dramatic increase in reported non-O157 STEC infections occurred between 2008 and 2010, during which time incidence increased nearly 3-fold, from 26 cases in 2008 to 76 cases in 2010.
The study looked at 74 clinical microbiological labs in Washington and 37 of those laboratories in 2010 were still not testing for non-O157:H7 types of E. coli. “Had all specimens been tested for both O157 and non-O157 STEC, we estimate that the incidence of non-O157 STEC would have been 60 percent of all reported STEC infections in 2010 rather than the reported 40 percent,” the study authors concluded.
Four serogroups accounted for more than 80 percent of non-O157 STEC case findings. They were E. coli O26, E. coli O103, E. coli O121 and E. coli O111.
The study authors wrote that because every reported STEC infection requires an epidemiologic investigation, enhanced detection and reporting of STEC infections will likely increase workloads for local communicable disease investigators and public health laboratories during a time when funding for public health is limited.




