The Centers for Disease Control (CDC) have just released a new update in the epidural prednisone steroid injection fungal meningitis outbreak associated with the New England Compounding Center (NECC). Now eleven people have died: one in Maryland, three in Michigan, six in Tennessee, and one in Virginia. So far, 119 people are sick in ten states.
Attorney Fred Pritzker is gathering evidence for wrongful death lawsuits against New England Compounding Center. “My experience with these kinds of cases is that patient safety was not the top priority. This company needs to be held accountable for the deaths and injuries caused by its product,” said Pritzker, who recently won $3,000,000 for a family who lost a loved one to an infectious disease.
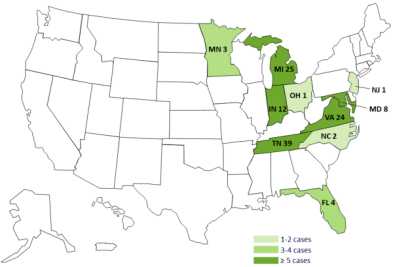
The case count is as follows by state: Florida (4), Indiana (12), Maryland (8 with 1 death), Michigan (25 with three deaths), Minnesota (3), New Jersey (1), North Carolina (2), Ohio (1), Tennessee (39 with six deaths), and Virginia (24 with 1 death). This outbreak is growing every day, since 13,000 people were injected with the potentially contaminated steroid. In Tennessee, which has the most cases and most deaths, Exserohilum has been identified as the fungus responsible for the illnesses.
One of the problems with this outbreak is that many of the patients have had very mild illnesses, unlike the typical symptoms of meningitis. Meningitis is inflammation of the meninges, which are the protective membranes covering the spinal cord and the brain. The symptoms of fungal meningitis can appear very gradually and can be very mild at first. The symptoms of meningitis include headache, fever, nausea, and neck stiffness. The additional symptoms of fungal meningitis can include confusion, dizziness, and discomfort from bright lights. In addition, patients may have symptoms of new weakness or numbness, increasing pain, and redness or swelling of the injection site. If you are experiencing any of these symptoms, see your doctor immediately.
All clinics that received the potentially contaminated steroid have been notified and those facilities should be actively contacting patients who received the drug. The injections from the recalled lot were given starting May 21, 2012, and symptoms typically develop within one to four weeks. The New England Compounding Center started recalling the product on September 26, 2012.