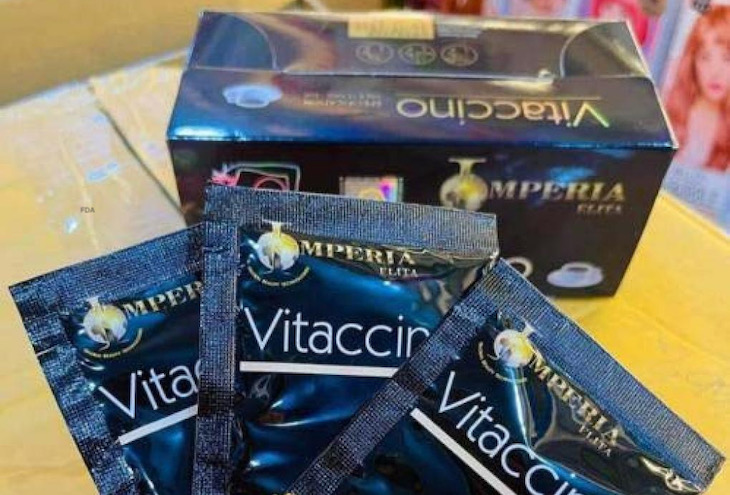
Dash Exclusive is voluntarily recalling all lots of Imperia Elita Vitaccino Coffee because it contains sibutramine and fluoxetine, two drugs that are not declared on the label. This product is a weight loss and anxiety dietary supplement. FDA analysis found the presence of these compounds in the product. The company has not received any reports of adverse reactions related to the use of this recalled product.
Sibutramine was an FDA-approved drug that was used as an appetite suppressant. It was withdrawn from the marketplace because of safety issues, including stroke, heart failure, and serious health risks, especially for people who have underlying heart disease. Sibutramine can significantly increase blood pressure and pulse rate.
Fluxoetine is an FDA-aprpved drug that is used to treat depression, obsessive compulsive disorder, bulilmina, and panic disorders. This drug has a warning label for suicidal thoughts and behaviors and anyone who takes it must be monitored by a prescriber. This product can also cause accumulation of high levels of serotonin in the body or neuroleptic malignant syndrome.
The presence of these two drugs in this product renders it an unapproved new drug for which safety and efficacy have not been established. That makes it subject to recall.
The recalled product is Imperia Elita Vitaccino Coffee that is packaged in a black rectangular black box. Each box contains fifteen sachets. The recall includes all lot numbers of this product. The supplement was disturbed nationwide in this country through the internet and on eBay.
On December 17, 2020, FDA issued a press release that warned consumers to avoid certain products found on Amazon, eBay, and other retailers due to hidden and potentially dangerous drug ingredients. Dash Xclusive is notifying it customers by e-messages on the eBay platform and is arranging for the return of all recalled products. If you purcahed this product, stop using it immediately and return it to the company.
If you have suffered an adverse reaction after using this product, see your doctor. You can then report the reaction to the FDA using their MedWatch Adverse Reaction Reporting Program.