The FDA has said it has recalled and destroyed products made with kratom, and warns consumers that the substance can be hazardous. The supplements it has collected and destroyed were distributed nationwide under the brand names Botany Bay, Enhance Your Life, and Divinity by Divinity Products Distribution of Grain Valley, Missouri. The company has agreed to stop selling all products containing kratom.
 The FDA is also encouraging all companies that sell products containing kratom to take steps to remove their products from the market and submit evidence to the FDA to evaluate them according to the regulatory pathway. Government officials are concerned about this product since the FDA has stated that 36 deaths are related to the use of the product.
The FDA is also encouraging all companies that sell products containing kratom to take steps to remove their products from the market and submit evidence to the FDA to evaluate them according to the regulatory pathway. Government officials are concerned about this product since the FDA has stated that 36 deaths are related to the use of the product.
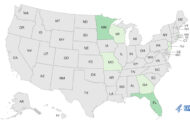
In addition, kratom products that have not been named are linked to a Salmonella I 4,[5],12:b:- outbreak that has sickened at last 28 people in 20 states. Eleven people are hospitalized because their illnesses are so severe.
FDA Commissioner Dr. Scott Gottlieb said in a statement, “The extensive scientific data we’ve evaluated about kratom provides conclusive evidence that compounds contained in kratom are opioids and are expected to have similar addictive effects as well as risks of abuse, overdose and, in some cases, death. At the same time, there’s no evidence to indicate that kratom is safe or effective for any medical use. To protect the public health, we’ll continue to affirm the risks associated with this product, warn consumers against its use and take aggressive enforcement action against kratom-containing products. We appreciate the cooperation of companies currently marketing any kratom product for human consumption to take swift action to remove these products from circulation to protect the public.”
Any dietary supplement that contains that compound needs to be the subject of a New Dietary Ingredient Notification demonstrating that the product is “reasonably expected to be safe.” To date, the FDA is not aware of any evidence of safety establishing that kratom is safe. This product should not be used to treat any medical conditions and should not be used as an alternative to prescription opioids.
Gottlieb added, “We know that some patients are using kratom because they believe it can help treat their opioid dependency, but there’s no reliable evidence to support kratom’s effectiveness for this use; and we’re deeply committed to making sure patients have access to safe, effective treatment options. There are three FDA-approved products that are safe and effective for the treatment of opioid use disorder and we encourage patients to seek advice from their health care professional and pursue treatment for addiction. Additionally, the FDA is taking new steps to bring new, safe and effective, FDA-approved therapies to the market for treatment of opioid use disorder. We understand that patients suffering from opioid addiction need access to effective treatment options.”
Anyone who has experienced an adverse reaction after taking a product can report it to the FDA. That agency has an online Safety Reporting Portal.