The FDA is warning consumers, retailers, and distributors about products from Pan-African Food Distributors doing business as East Africa Boutique because they were held under insanitary conditions and could be contaminated with filth. These items include infant nutritional cereals, baking ingredients, and other food products. The products may or may not have a label with the firm name. The warning was updated on June 3, 2025 to include cosmetics. Products that are held under insanitary conditions could pose a serious health risk and could lead to serious illness, including leptospirosis, hantavirus infection, salmonellosis, yersiniosis, E. coli infections, and rat-bite fever. You can see the long list of products, along with package sizes, lot codes, and expiration dates at the … [Read more...]
Hidden Romaine E. coli O157:H7 Outbreak Sickened 98 in 2024
A hidden romaine E. coli O157:H7 outbreak sickened at least 98 people in 2024, hospitalized 36, and killed one person, according to an FDA report that was obtained by NBC News. This outbreak was not publicized by the FDA. Officials in Missouri first alerted the FDA to the issue when an E. coli O157:H7 outbreak sickened people who were served food by a caterer in Missouri. Those events took plate on November 6 and November 8, 2024. The case count by state was: Arkansas (2), Colorado (1), Illinois (7), Indiana (8), Kansas (1), Kentucky (1), Missouri (50), Montana (1), North Dakota (2), Nebraska (3), Ohio (8), Pennsylvania (1), South Dakota (1), Tennessee (1), and Wisconsin (2). Illness onset dates ranged from November 4, 2025 to November 30, 2025. The patient age range was from 4 to … [Read more...]
Protect Yourself in the Wake of Cuts to FDA, USDA, and CDC
Learn how to protect yourself in the wake of cuts to FDA, USDA, and CDC. Today we learned that many scientists and food safety experts have lost their jobs at those agencies. What does that mean to you and your family and how can you protect yourself? You have always been the final piece of the puzzle for food safety. Contaminated food has always been sold; in fact, the government has allowed chicken to be sold when it is contaminated with a certain level of Salmonella, and recalls often come after people have been sickened by contaminated food. So if you are already following food safety precautions you are ahead of the game. First of all, always follow the rule of Clean, Separate, Cook, and Chill. Wash your hands before you start cooking. Start with a clean kitchen and … [Read more...]
FDA Releases Allergen, Food Safety, and Plant Based Labels
FDA releases allergen, food safety, and plant based labeling guidelines to help industry understand and comply with regulations. The food safety guidelines cover low moisture ready to eat foods. Food Allergens This guidance covers food allergens, including questions and answers about food allergen labeling requirements, the labeling of tree nuts, sesame, milk, eggs, incidental additives, highly refined oils, dietary supplement products, and certain specific packing and labeling situations, such as individual units within a multiunit package. The FD&C Act requires that any food that is made from two or more ingredients must declare each ingredient by its common or usual name. But consumers may not be familiar with those names, and may not recognize that the ingredients contain … [Read more...]
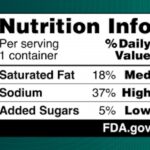
FDA Proposes Updates to Front of Package Nutrition Labels
The FDA is proposing updates to front of package nutrition labels to help consumers make healthier choices. The rule would require a front-of-package (FOP) nutrition label on most packaged foods. These labels would give consumers more information at a glance. This label is called the "nutrition info box," and it would complement the longer label already on these products that is usually found on the back or side panel. The difference is that the new label would amplify the most important nutrient information about saturated fat, sodium, and added sugars. The contents would be labeled "Low," Med," or "High," to give consumers information at a glance. These descriptive terms follow federal dietary recommendations and may encourage consumers to choose more nutrient-dense … [Read more...]