Primus is voluntarily recalling all lots within a certain expiration date of Limbrel, a prescription medical food, because of rare but serious and reversible adverse events. There have been 30 adverse events reported to the government between January 7, 2007 and November 9, 2017. Elevated liver function tests or acute hypersensitivity pneumonitis have been associated with the use of these products.
These conditions occurred in rare cases with varying degrees of severity in patients taking this product for the first time within the first few weeks. Patients may not notice the adverse effects until they consult with their doctor or until they need to be hospitalized. No deaths have been reported, and all adverse effects resolved without any residual problems after the patient stopped taking this product.
Primus has retained independent medical and former senior FDA safety experts to conduct an investigation into these events and the ingredients in Limbrel. The statement posted on the FDA website reads, “It is the opinion of these experts based on a thorough review of the medical literature, adverse event reports to FDA, and FDA’s health hazard evaluation that there is no basis on which to conclude that Limbrel potentially causes life-threatening adverse effects, and that none of the reported adverse events show liver failure or respiratory failure. Nonetheless, in an effort to cooperate with FDA, Primus voluntarily ceased its promotion and distribution of Limbrel on December 21, 2017, and is now recalling Limbrel as FDA has requested.”
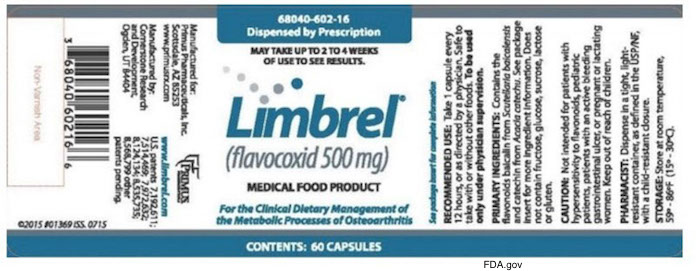
These are the recalled products being recalled within expiry: Limbrel (flavocoxid) 250 mg capsules, Product Identity Number 68040-601-16; Limbrel250 (250 mg flavocoxid with 50 mg citrated zinc bisglycinate) capsules, Product Identity Number 68040-605-16; Limbrel (flavocoxid) 500 mg capsules, Product Identity Number 68040-602-16; and Limbrel500 (500 mg flavocoxid with 50 mg citrated zinc bisglycinate) capsules, Product Identity Number 68040-606-16. These products were marketed as a medical food available by prescription for patients under the supervision of a doctor for the dietary management of osteoarthritis.
If you have these products, contact Primus and your doctor to return them. And contact your doctor if you have experienced any adverse event that may be related to taking this product. Adverse reactions can be reported to the FDA’s MedWatch Adverse Event Reporting program.