Dr. Reddy’s Laboratories has initiated a voluntary recall of over the counter and prescription Dr. Reddy’s Ranitidine products because they are contaminated with N-Nitrosodimethylamine (NDMA) above levels established by the FDA. That ingredient is classified as a possible human carcinogen. It is also an environmental contaminant. There have been no reports of adverse events related to this recall.
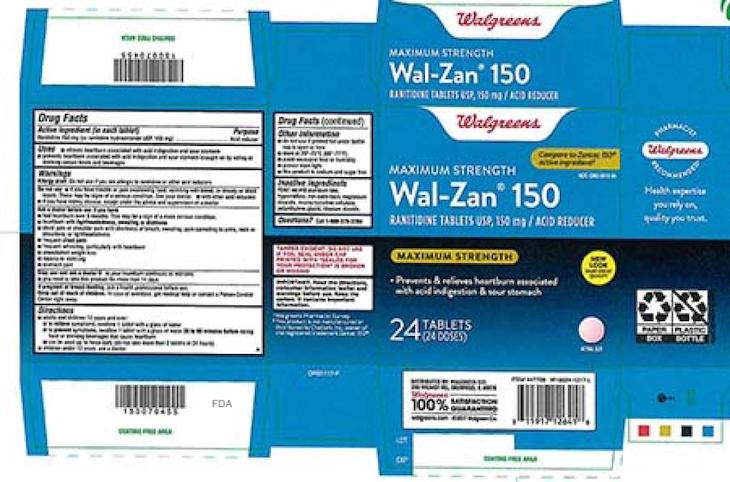
This recall follows the FDA’s caution note alerting patients and health care professionals that NCMA was found in some samples of ranitidine. Ranitidine is an over the counter drug used to relieve heartburn that is associated with acid indigestion. They are also used to prevent heartburn associated with indigestion. The prescription version of this product is used for treatment of ulcers.
The recall affects all quantities of these products that are within the expiration date. You can see the long list of recalled products, along with the stores where they were sold, the package sizes, and UPC numbers, at the FDA web site. Some stores that may have carried these products include Kroger, Walmart, Walgreens, CVS, Sam’s Club, Target, and Thirty Madison, among others. The products that are recalled are all Dr. Reddy’s Ranitidine tablets in various sizes.
If you bought any of these products, stop using them. Throw them away in a secure garbage can, or take them back to the place of purchase for a full refund.
If you have experienced problems when taking these products, contact your doctor. You can also fill out an adverse event report with the FDA, using the FDA’s Medwatch Adverse Event Reporting Program.




