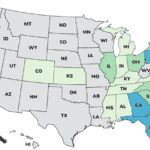
The Bedner cucumber Salmonella outbreak has ended with 69 people sick in 21 states, according to the CDC. That is an increase of 24 more patients and three more states since the last update was issued on May 30, 2025. The new states are Maryland, Mississippi, and New Jersey. This outbreak is over. The patient case count per state is: Alabama (1), California (1), Colorado (1), Florida (7), Georgia (10), Illinois (5), Indiana (1), Kansas (1), Kentucky (1), Massachusetts (3), Maryland (1), Michigan (2), Mississippi (1), North Carolina (4), New Jersey (3), New York (4), Ohio (6), Pennsylvania (7), South Carolina (6), Tennessee (1), and Virginia (3). Illnesses started on dates ranging from April 2, 2025 to May 29, 2025. The patient age range is from 1 to 89 years. Of the 60 people who … [Read more...]
Safetussin Max Strength Blister Packs Recalled For Packaging
Safetussin Max Strength Blister Packs for cough, colds, and flu are being recalled for violating the federal regulations for child resistant packaging. This poses a poisoning risk if children get ahold of the product. No illnesses or injuries have been reported to date in connection with this issue. The distributor is Kramer Laboratories of Bridgewater, New Jersey, a subsidiary of Arcadia Consumer Healthcare of Bridgewater, New Jersey. The product was manufactured in India. This product was sold at HEB, Harris-Teeter, and other regional grocery stores and independently owned pharmacies nationwide from July 2024 through March 2025 for about $11.00. The Safetussin over-the-counter cold medicine contains acetaminophen, which must be in child-resistant packaging as required by the … [Read more...]
Premium Quality Goods Beef Tallow Recalled For No Inspection
Premium Quality Goods Beef Tallow is being recalled for lack of federal inspection. The tallow was processed, packaged, and labeled on behalf of Lady May Tallow by Common Sense Soap of North Walpole, New Hampshire. Common Sense Soap does not possess a federal grant of inspection to produce food products. The tallow contains a nutrition facts label, which may lead people to believe that the product is safe for human consumption, but Common Sense Soap does not have a federal grant of inspection and is not authorized to product products intended for human consumption. No confirmed reports of adverse reactions have been received to date. These products were produced between October 16, 2024 and February 6, 2025. They are all Premium Quality Goods brand. They include: 24 fluid … [Read more...]
Number One Outbreak of 2024 Was Bedner Cucumber Salmonella
The number one outbreak of 2024 was the Bedner cucumber Salmonella outbreak that sickened at least 551 people in 34 states and the District of Columbia. The cucumbers were also sold by Thomas Produce Company. That outbreak ended in August 2024. The case count by state was: Alabama (6), Arkansas (1), California (1), Connecticut (8), Delaware (3), Florida (60), Georgia (48), Illinois (9), Indiana (4), Iowa (5), Kentucky (20), Maine (4), Maryland (17), Massachusetts (11), Michigan (12), Minnesota (10), Mississippi (2), Missouri (4), Nevada (1), New Jersey (22), New York (69), North Carolina (27), Ohio (20), Oklahoma (2), Pennsylvania (68), Rhode Island (8), South Carolina (22), Tennessee (22), Texas (2), Vermont (2), Virginia (48), Washington (1), West Virginia (7), Wisconsin (4), and … [Read more...]
Top 10 2024 Outbreaks: Number Two is WanaBana Lead Poisoning
The top 10 2024 outbreak list continues with number nine: the WanaBana lead illness outbreak. There are at least 500 children sick after eating WanaBana apple sauce pouches, along with Weis cinnamon apple sauce and Schnucks applesauce. The FDA reported 90 children sick, but since these outbreaks are usually much larger than the known cases, we decided to go with the larger CDC number. Each agency used different criteria to identify possible patients. There are 93 confirmed cases of lead poisoning, 233 probable cases, and 28 suspect cases. The patients live in these 41 states: Alabama, Arkansas, California, Colorado, Connecticut, Florida, Georgia, Iowa, Idaho, Illinois, Indiana, Kansas, Kentucky, Louisiana, Massachusetts, Maine, Michigan, Minnesota, Missouri, Mississippi, Montana, … [Read more...]
Top 10 2024 Outbreaks: Number Nine is Milo’s Eggs Salmonella
The top 10 2024 outbreak list continues with the number nine outbreak: the Milo's eggs Salmonella outbreak, which sickened at least 93 people in 12 states. There were 34 people hospitalized because they were so sick. There were several recalls issued in relation to this outbreak. And it's wasn't only chicken eggs that were implicated in this outbreak; duck eggs were included in the recall. The case count by state was: Arizona (1), California (5), Colorado (1), Illinois (12), Iowa (2), Michigan (3), Minnesota (5), New York (1), Oregon (1), Utah (1), Virginia (1), and Wisconsin (60). The patient age range is from 2 to 88 years. Thirty-four people have been hospitalized because they are so sick. That’s a hospitalization rate of 37%, which is much higher than the typical 20% … [Read more...]
Some VidaSlim Products Recalled For Containing Yellow Oleander
Some VidaSlim products are being recalled because they may contain yellow oleander. Yellow oleander is a toxic plant that can cause serious illness and death. Yellow oleander can cause neurologic, gastrointestinal, and cardiovascular adverse health effects that can be severe or fatal. No illnesses have been reported to the company to date in connection with the consumption of these VidaSlim products. The recalled products include VidaSlim 90-day (Original Root, Root Plus, and Root Capsules), VidaSlim 30-day (Original Root, Root Plus, and Root Capsules), VidaSlim 7-day Sample Size (Original Root, Root Plus, and Root Capsules), VidaSlim Hot Body Brew (Strawberry and Peach flavors), and VidaSlim 7-day Sample Pouches for Original Root, Root Plus, and Root Capsules. Samples of some of … [Read more...]
SunFed Cucumber Salmonella Outbreak Sickens 68 in 19 States
A SunFed cucumber Salmonella outbreak has sickened at least 68 people in 19 states, according to the CDC. Eighteen of those patients have been hospitalized. No deaths have occurred. The case count by state is: Alaska (1), California (1), Colorado (8), Iowa (2), Illinois (2), Massachusetts (5), Montana (16), Nebraska (1), New Jersey (1), New York (1), Ohio (1), Oregon (7), Pennsylvania (1), South Dakota (4), Texas (5), Utah (2), Washington (5), Wisconsin (3), and Wyoming (2). Illness onset dates range from October 12, 2024 to November 16, 2024. The patient age range is from less than one year to 98 years. Of the 50 people who gave information to investigators, 18 have been hospitalized, for a hospitalization rate of 36%, which is very high for a Salmonella outbreak. Of the 33 … [Read more...]
Gaines Family Farmstead Chicken Chips Recalled For Salmonella
Gaines Family Farmstead Chicken Chips are being recalled for possible Salmonella contamination. No illnesses have been reported to the company to date in connection with this issue. The recalling firm is Gaines Pet Treats of Birmingham, Alabama. There are 204 bags of this product included in this recall. The recalled item is Gaines Family Farmstead Chicken Chips that are packaged in purple 5 ounce bags with a picture of a chicken on the front. The bag is marked on the back side with the lot number 20061124 and the expiration date 12/11/25. This is the only lot number that has been identified as potentially contaminated, The recall was triggered when a third party tested and found contamination in a related, unreleased lot of the same product. This item was distributed online and … [Read more...]