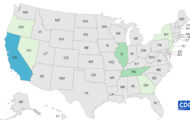
An FDA warning letter to Sprouts Unlimited, located in Marion, Iowa, details information about problems at the company’s facility in the wake of a 2019 E. coli O103 outbreak in Iowa linked to their raw clover sprouts served at Jimmy John’s restaurants. The FDA has determined that the company’s clover sprouts are adulterated in that they “bear or contain an added poisonous or deleterious substance which may render them injurious to health.”

The FDA inspected the Sprouts Unlimited facility from December 31, 2019 to January 9, 2020. Inspectors documented “numerous serious violations of the Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption regulation (PSR), Title 21, Code of Federal Regulations, Part 112 (21 CFR Part 112) that may have resulted in the contamination of your sprouts with human pathogens. Accordingly, your mung bean, red bean, alfalfa, clover, broccoli, onion, radish, pea, and lentil sprout products, and any combination thereof, are further adulterated within the meaning of section 402(a)(4) of the Act [21 U.S.C. § 342(a)(4)], in that they were prepared, packed, or held under insanitary conditions whereby they may have become contaminated with filth or been rendered injurious to health.”
Analysis of clover sprouts and spent sprout irrigation water collected by the company found a strain of E. coli O103 that is highly related to the outbreak strain. Analysis of samples taken on December 23, 2019 found that 15 of 16 sprout samples and one of the water samples yielded E. coli O103 that whole genome sequencing determined is from the same source as the outbreak strain.
The FDA found that there were significant violations of the Produce Safety Rule, part of the 2011 Food Safety Modernization Act, at the Sprouts Unlimited facility. The company did not test sprout irrigation water from each production batch or collect water samples for each production batch of sprouts. They also moved trays of growing sprouts, so the government doesn’t know how many production batches were planted during a given timeframe. No supporting documents were provided for the claim that a lot number system for sprouts has been developed.
The company does not hold all product from entering commerce before receiving results from the spent sprout irrigation water tests. Moving trays of sprouts, again, makes it impossible to determine which trays have been tested.
Sprouts Unlimited did not take corrective action when environmental samples tested positive for Listeria monocytogenes. When the company was notified that an environmental sample collected in September 2019 tested positive for Listeria, they did not conduct finished product testing of sprouts. Again, racks of sprouts were moved without keeping track, which makes it difficult to determine which sprouts were in contact with the contaminated surfaces.
Food contact surfaces that are used to grow, harvest, pack, and hold sprouts are not cleaned and sanitized. After the notification in December 2019 act samples and water tested positive for E. coli O103, they did not clean or sanitize sprout growing trays before reusing them; they were only rinsed off.
If the company does not make corrective action, the FDA can seize product or enact a court injunction. Sprouts Unlimited must respond within 15 working days of the letter.