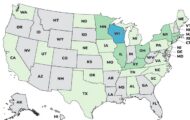
Why Not Natural Moringa capsules are being recalled for possible Salmonella contamination. There is a Salmonella outbreak linked to moringa powder that has sickened at least 65 people in 28 states and hospitalized 14. The FDA investigation states that these capsules are linked to this outbreak.

The recalled product is Why Not Natural Organic Moringa Capsules that is a dietary supplement. They were sold nationwide through mail orders on the company’s web site and third party on-line retailers including Amazon. The product was available for purchase from July 2025 to January 2026.
The moringa powder is packaged in a 120 capsule bottle m marked with lot number A25G051 on the bottom. The expiration date of 07/2028 is stamped on the bottom of the bottle. No pictures of the recalled product were provided in the recall notice.
The FDA traceback investigation into the outbreak found a common manufacturer between Live It Up Super Greens, which was named in the original outbreak notice on January 14, 2025, and these capsules. At that time, 45 people were sick. Distribution of the product has been suspended while the FDA and the company continue their investigation into the outbreak.
If you purchased this product, do not consume it. You can throw it away in a secure trash can after double bagging the bottle so other people can’t see it, or you can contact the company for a full refund.
If you ate these capsules, monitor your health for the symptoms of a Salmonella food poisoning infection for the next week. If you do get sick, see your doctor.