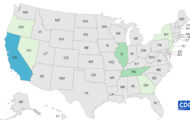
Kingsway Trading Inc. is recalling Xanthium & Siler Combo (Bi Yan Pian) Dietary Supplement because it contains banned ephedra alkaloids. This compound can cause heart attack, stroke, and death. The recall notice states, "these risks are unreasonable in light of any benefits that may result from the use of these products under their labeled conditions of use, or under ordinary conditions of use if the labeling is silent." The product was distributed to Massachusetts, New Jersey, New York, Illinois, Maryland, Florida, Missouri, Texas, Indiana, Georgia, Delaware, Colorado, Virginia, Pennsylvania, Connecticut, Oregon, Virginia, and Arizona through oriental herb stores, acupuncture clinics, and Oriental supermarkets. No illnesses have been reported to date. The recalled product is … [Read more...]
ABX Weight Loss Supplement Contains Hidden Drug
The FDA is advising consumers not to purchase or use ABX Weight Loss, a product promoted for weight loss, because it contains sibutramine, a controlled substance. There is no word on whether or not this product has been associated with any adverse effects or illnesses. Sibutramine was removed from the market in October 2010 because it substantially increases blood pressure and/or pulse rate in some people and may be a significant risk for people with heart disease. It may also interact in life-threatening ways with other medications. The product was sold at www.zxtbeepollenpills.com and may have been sold in some retail stores. If you purchased this product, do not use it. Take it to the drug disposal center in your area or return it to the place of purchase for a refund. You can … [Read more...]
FDA Issues Warning Against ENVY BP Supplement
The Food and Drug Administration (FDA) is warning consumers not to purchase or use ENVY BP, a product promoted and sold for weight loss on several websites, including Purely Simple Weight Loss. This product may also have been sold in some retail stores. FDA analysis confirmed that ENVY BP contains sibutramine, a controlled substance that was withdrawn from the market in October 2010 for health threats. Sibutramine substantially increases blood pressure and/or pulse rate in some patients and can be very risky for anyone who has coronary artery disease, congestive heart failure, arrhythmias, or stroke. This product can also interact in life-threatening ways with other meds. This is one in a long line of recalls for weight loss supplements that contain sibutramine. The FDA has warned … [Read more...]
Smart Lipo Recalled for Undeclared, Banned Drugs
SmartLipo365 of Dallas is recalling all lots of Smart Lipo capsules because they have been found to contain contain undeclared, banned drugs. Consumers who have purchased these products should not use them as they could cause serious, life-threatening health problems. All 800, 900 and 950 mg capsules are being recalled after the U. S. Food and Drug Administration (FDA) conducted an analysis and found them to contain sibutramine, desmethylsibutramine, and phenolphthalein which are not declared on the label. Sibutramine is banned drug. Before it was removed from the market in 2010 for posing health risks to patients with a history of coronary artery disease, congestive heart failure, arrhythmias or stroke, it was used as an appetite suppressant. Phenolphthalein is a banned drug … [Read more...]
After Illness Reported, Lipo Escultura Recalled for Undeclared, Banned Ingredients
Lipo Escultura Corp. of Brooklyn dba JAT Productos Naturales Corp., and JAT Natural Products Corp. is recalling all Lipo Escultura because it contains banned and undeclared ingredients--sibutramine and diclofenac. Consumers who have purchased this product should not use it as it could cause serious injury. One illness has already been reported. Sibutramine is an appetite suppressant that was removed from the market for safety reasons. It causes increased blood pressure and/or pulse rate in some patients and could adversely effect patients with a history of coronary artery disease, congestive heart failure, arrhythmias, or stroke. Diclofenac is a non-steroidal anti-inflammatory drug (commonly referred to as NSAIDs). NSAIDS can also increase the risk of cardiovascular events, such as … [Read more...]