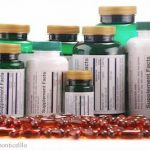
New York Attorney General Eric T. Schneiderman has asked GNC, Target, Walmart, and Walgreens to stop selling their store brand herbal supplements immediately because of mislabeling. Cease and desist letters sent to the companies yesterday say tests on the products found 79 percent did not contain plant species identified on the label. And some contained contaminants not identified on product labels. Supplements do not have to undergo a premarket evaluation by the U.S. Food and Drug Administration (FDA) before they are sold. Manufacturers are responsible for ensuring that their products are safe and accurately labeled. Unfortunately, not all of them do. In fact, many supplements, especially those sold as weight loss or body building supplements, contain undeclared ingredients that … [Read more...]
Study Reveals Misrepresentation of Shrimp in Markets
A study conducted by Oceana has revealed misrepresentation of America's favorite seafood - shrimp - across the country. DNA testing has confirmed that 30% of shrimp products purchased from 111 grocery stores and restaurants were misrepresented. Misrepresentation means that the shrimp were mislabeled, with one species substituted for another; misleading, with farmed species labeled as "Gulf shrimp", for instance; or mixed/mystery, with species commingled among bagged shrimp. The misrepresentation was found everywhere in the country. The highest rates of misrepresentation were in New York at 43%, 33% in Washington, D.C., and 30% in the Gulf of Mexico region. This problem makes it difficult for consumers to make informed choices about the shrimp they purchase in stores and in … [Read more...]
Ibuprofen and Oxcarbazepine Tablets Recalled for Incorrect Labeling
American health packaging is recalling Ibuprofen Tablet in a hospital unit dose presentation that may contain Oxcarbazepine Tablets. The recall is the result of a mislabeled inner unit dose blister packaging which could mean patients get ibuprofen instead of their scheduled dose of oxcarbazepine. There was one customer complaint that resulted in the investigation and recall. There have not been any adverse events reported to date. Oxcarbazepine is used for treating seizures in epilepsy patients. These are the affected products: Cartons of 100 count (10x10) Hospital Unit Dose blisters of AHP Ibuprofen Tablets, USP, 600 mg, with outer carton NDC#: 68084-703-01 and individual dose NDC#: 68084-703-11, Lot #142588, Expiration Date, 01/2016. The drug product can be identified by physical … [Read more...]