Aurobindo Pharma USA is voluntarily recalling one lot of Gabapentin Capsules because the bottles contain some empty capsules. Empty capsules could result in patients missing doses, which would result in adverse health consequences that range from no effect, short term withdrawal effect, or status epilepticus (long period seizures) that could be life-threatening. No reports of adverse health events have been reported to date. Gabapentin is used for the treatment of epilepsy and for the management of postherpetic neuralgia (pain after shingles). The product is sold in 300 mg 100-count bottles. The lot number is GESB14011-A, the expiration date is 12/2015, and the NDC is 16714-662-01. The product has the imprint D on the yellow cap and 03 on yellow body with black edible ink. The product … [Read more...]
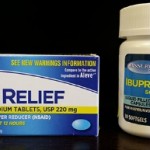
Assured Naproxen Sodium Tablets Recalled
Contract Packaging Resources, a drug packaging company is voluntary recalling 11,640 boxes of Assured brand Naproxen Sodium tablets because some cartons contain bottles of Ibuprofen, a different pain reliever. Consumers who must avoid using Ibuprofen may have inadvertently purchased Ibuprofen 200 mg soft gels, believing it was Naproxen Sodium 220 mg tablets. Allergic reactions from this mix up can range from mild irritation or hives to live-threatneing anaphylaxis. No reports of adverse reactions have been received to date. The recalled product is boxes of Assured brand Naproxen Sodium Tablets 220 mg, 15 count. The lot number is FH4102A, the SKU number is 122368, and the UPC number is 639277223685. The products were distributed nationwide to Dollar Tree stores and sold via Dollar Tree … [Read more...]
Ibuprofen and Oxcarbazepine Tablets Recalled for Incorrect Labeling
American health packaging is recalling Ibuprofen Tablet in a hospital unit dose presentation that may contain Oxcarbazepine Tablets. The recall is the result of a mislabeled inner unit dose blister packaging which could mean patients get ibuprofen instead of their scheduled dose of oxcarbazepine. There was one customer complaint that resulted in the investigation and recall. There have not been any adverse events reported to date. Oxcarbazepine is used for treating seizures in epilepsy patients. These are the affected products: Cartons of 100 count (10x10) Hospital Unit Dose blisters of AHP Ibuprofen Tablets, USP, 600 mg, with outer carton NDC#: 68084-703-01 and individual dose NDC#: 68084-703-11, Lot #142588, Expiration Date, 01/2016. The drug product can be identified by physical … [Read more...]