SNI National is recalling all Kratom products because they contain Kratom (Mitragyna Speciosa), which is a new ingredient for which there is “inadequate information to provide reasonable assurance that such ingredient does not present a significant or unreasonable risk of illness or injury.” Scientific literature shows serious concerns about the toxicity of Kratom in organ systems. Consumption of these products may lead to “respiratory depression, nervousness, agitation, aggression, sleeplessness, hallucinations, delusions, tremors, loss of libido, constipation, skin hyperpigmentation, nausea, vomiting, and severe withdrawal signs and symptoms,” according to the FDA release.
The primary ingredient doesn’t fall under the Federal Food, Drug, and Cosmetic Act as having all the information necessary to deem it a safe product. No complaints, illnesses, or adverse effects have been reported to date.

The recalled ingredients include Kratom XL 4 Pack, Maeng Da Kratom 10 Pack, Max Kratom 20 Pack, and Bali Kratom 40 Pack. SNI didn’t manufacture these products, bur re-packaged them and re-labeled them for sale to wholesalers and distributors.
The products are packaged in clamshell, zip sealed packets and green pill bottles, in 4, 10, 20, and 40 count. They can be identified by bright green packaging and label that states it contains Kratom. You can see product labels at the FDA web site.
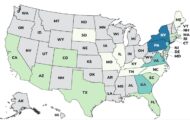
They were distributed in Alabama, California, Illinois, Missouri, Kentucky, Florida, Oklahoma, Idaho, Colorado, Wisconsin, Massachusetts, and Ohio and may have been distributed further around the country. SNI National has completely terminated distribution.
If you purchased any of these products, do not take them. Adverse reactions or quality problems can be reported to the FDA’s MedWatch Adverse Event Reporting program.