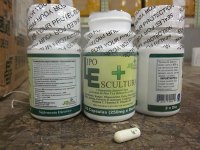
 Lipo Escultura Corp. of Brooklyn dba JAT Productos Naturales Corp., and JAT Natural Products Corp. is recalling all Lipo Escultura because it contains banned and undeclared ingredients–sibutramine and diclofenac. Consumers who have purchased this product should not use it as it could cause serious injury. One illness has already been reported.
Lipo Escultura Corp. of Brooklyn dba JAT Productos Naturales Corp., and JAT Natural Products Corp. is recalling all Lipo Escultura because it contains banned and undeclared ingredients–sibutramine and diclofenac. Consumers who have purchased this product should not use it as it could cause serious injury. One illness has already been reported.
Sibutramine is an appetite suppressant that was removed from the market for safety reasons. It causes increased blood pressure and/or pulse rate in some patients and could adversely effect patients with a history of coronary artery disease, congestive heart failure, arrhythmias, or stroke.
Diclofenac is a non-steroidal anti-inflammatory drug (commonly referred to as NSAIDs). NSAIDS can also increase the risk of cardiovascular events, such as heart attack and stroked, as well as serious gastrointestinal damage, including bleeding, ulceration, and fatal perforation of the stomach and intestines.
The recalled product is marketed as a weight loss dietary supplement and is packaged in a white plastic bottle with green and lime labeling with white capsules. Products were sold/distributed nationwide from the website www.lipoesculturatreatment.com, and from a retail store in Brooklyn.
.




