Federal health officials are investigating a possible link between Similac, EleCare, and Alimentum powdered instant baby formulas and a Cronobacter and Salmonella outbreak. All three products were made at Abbott Nutrition’s facility in Sturgis, MI.
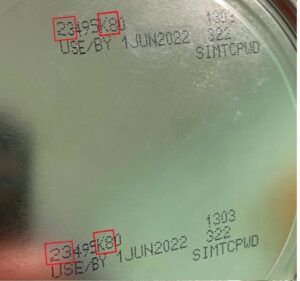
The FDA is advising consumers to avoid buying or using these powdered infant formula products which have the following identification on the bottom of the can.
- the first two digits of the code are 22 through 37 and
- the code on the container contains K8, SH, or Z2, and
- the expiration date is 4-1-2022 (APR 2022) or later.
The FDA and the Centers for Disease Control and Prevention (CDC) are investigating four illnesses among infants who consumed products made at Abbott Nutrition’s Sturgis, MI facility. The illnesses were reported from three states, MN (1), OH (1), TX (2) from September 6, 2021, to December 18, 2021.
One of the infants developed a Salmonella Newport infection, three others developed Cronobacter sakazakii infections. All four were hospitalized and Cronobacter may have contributed to a death in one case, according to the FDA.
Cronobacter is a bacteria that can live on dry foods such as herbal teas, powdered milk, and baby formula. Illnesses, though rare, can be deadly for newborns, according to the CDC. That’s because Cronobacter can cause sepsis and meningitis.
For infants, symptoms of these infections include blotchy skin, a pinprick rash, high fever, shivering, fatigue, rapid breathing/difficulty breathing, unusual grunting sounds, refusal to feed, stiff jerky movements or a very floppy body, cold hands and feet, irritability, and diarrhea.
If your infant has these symptoms, seek medical help right away.
During an onsite inspection at Abbott Nutrition’s Sturgis plant, the FDA collected environmental swabs and several of them were positive for Cronobacter. In its advisory, the agency stated that it found “adverse inspectional observations” and discovered through a review of the firm’s records that the company had found environmental contamination with Cronobacter sakazakii and destroyed product due to the presence of Cronobacter.
Shortly after the FDA issued the advisory, Abbott issued a baby formula recall.
Update (Feb. 18, 2022): Abbott also contacted Food Poisoning Bulletin with the following comment, “We value the trust parents place in us for high quality and safe nutrition and we’ll do whatever it takes to keep that trust and resolve this situation.”





