Attorney Fred Pritzker, the author of this post, is representing Traci (TM) and over 40 patients harmed in the national fungal meningitis outbreak. In the weeks since discovery of the massive fungal meningitis outbreak traced to the New England Compounding Center in Massachusetts, much of the news has centered on the company’s long history of corporate malfeasance including its disregard of sterility tests, preparation of medication in unsanitary conditions and systematic violation of its pharmacy license. The company’s willful (and potentially criminal conduct) was abetted by regulatory inaction of such magnitude that some of the officials charged with overseeing the company have already been fired. As they always do, the lurid details and public ire will fade from memory as the … [Read more...]
Traci’s Story: The Human Cost of Corporate Greed and Government Malfeasance in the New England Compounding Center Drug Poisoning Outbreak
Fungal Meningitis Outbreak: Pharmacy Board Chief Fired, Case Count Mounts
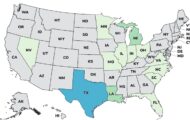
The pharmacy board chief in Massachusetts, where tainted injectable steroids that have sickened 424 people in 19 states and caused 31 fatalities were produced, has been fired. James Coffey, director of the Massachusetts Board of Registration in Pharmacy, did not act on a complaint that the New England Compounding Center (NECC) was functioning like a drug manufacturer. Compounding pharmacies generally custom-mix drugs for a specific geographic area. NECC, based in Framingham, Mass., prepared the steroid, preservative-free methylprednisolone acetate, for nationwide distribution. Compounding pharmacies are not regulated like drug manufacturers. So it wasn't until after the outbreak began that inspectors from the U.S. Food and Drug Administration (FDA) looked inside the facility where … [Read more...]
Meningitis Outbreak Prompts Ameridose To Recall All Drugs
Ameridose LLC, the sister company to the compounding pharmacy linked to a fungal meningitis outbreak that has sickened 377 in 28 states and killed nine people, announced a recall of all of its products yesterday after federal inspectors discovered inadequacies in its sterility testing procedures. The Westborough, Mass.- based company, which distributes drugs to thousands of hospitals nationwide, notified its customers of the recall in a letter. “During the course of its on-going inspection of our facility, FDA has notified Ameridose that it will be seeking improvements in Ameridose’s sterility testing process. Ameridose and FDA agree that the use of injectible products that are not sterile can represent a serious hazard to health and could lead to life-threatening injuries and/or … [Read more...]