A new cyclospora outbreak has been added to the FDA’s CORE Outbreak Investigation Table, while two other outbreaks have ended unsolved. The new cyclospora outbreak has sickened at least six people. The food responsible has not yet been identified. And traceback has been initiated.
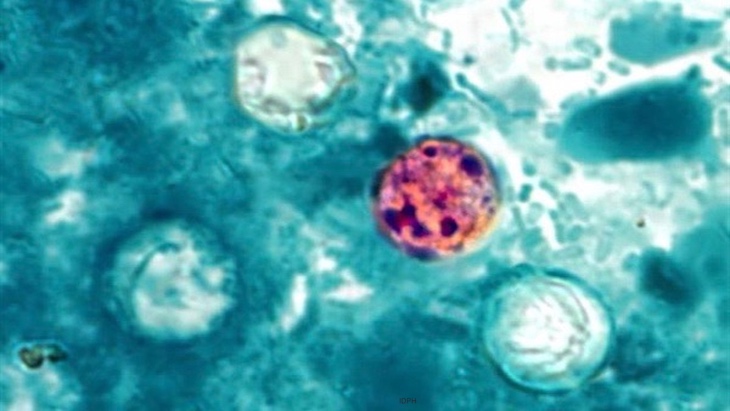
This joins the other cyclospora outbreak that was announced last week, with at least 60 sick and no food, again, identified. Traceback has been initiated in that investigation, and samples have been collected for analysis.
The two outbreaks that have ended are the Salmonella Paratyphi B var. L(+) tartrate+ that sickened at least 14 people. Although traceback was conducted, no food was identified. And a Listeria monocytogenes outbreak that sickened at least 12 people has also ended unsolved. The only action taken was traceback.
The active outbreaks on the table include the Daily Harvest French Lentil + Leek Crumbles illnesses. There have been more than 320 reports of illness received by the FDA. Tara flour has been named as a suspect, but no toxin, pathogen, or chemical has been found that may have caused the symptoms of jaundice, itching, dark urine, fatigue, severe abdominal pain, fever, nausea, and body aches.
The deadly Listeria monocytogenes outbreak linked to recalled Big Olaf ice cream is still ongoing. There are 23 illnesses in that outbreak, 22 hospitalizations, one death, and one fetal loss. Listeria was found in environmental samples taken art the Big Olaf facility in Florida, and the pathogen was also found in the ice cream.
And the hepatitis A outbreak linked to recalled organic HEB and FreshKampo strawberries is ongoing. There are 18 people sick in three states, and 13 people have been hospitalized.
The Salmonella Braenderup outbreak that is not yet linked to any food is still active. The case count has increased from 70 to 74 people sick. Traceback has been initiated, but that’s all.
Finally, the illnesses that may be associated with dry cereal are still under investigation. Three have been 558 adverse event report received by the FDA. An on-site inspection, sample collection, and analysis have all been conducted by the FDA.

If you have been sickened with a food poisoning infection, please contact our experienced attorneys for help with a possible lawsuit at 1-888-377-8900 or text us at 612-261-0856. Our firm represents clients in lawsuits against grocery stores and food processors.




