The FDA is warning parents about the dangers of benzocaine and lidocaine in products used for treating teething pain in children. These topical medications offer little to no benefit and are associated with serious risks. The products include creams, gels, and homeopathic teething tablets. Benzocaine is a local anesthetic that temporarily numbs. It is the active ingredient in several nonprescription oral health care products, including Anbesol, Cepacol, Chloraseptic, HurriCaine, Orabase, Orajel, and Topex. These products should not be used for teething pain in children because they can be dangerous. Using benzocaine products can lead to a serious, and sometimes fatal, condition called methemoglobinemia, in which the oxygen-carrying capacity of red blood cells is greatly … [Read more...]
FDA Warns Against Teething Necklaces and Bracelets
The FDA is warning parents about the risk of teething necklaces, bracelets, and other jewelry that is marketed for relieving teething pain or providing sensory stimulation because they could be choking or strangulation devices. The safety of teething jewelry has not been determined. These products are not the same as teething rings or teethers, which are made of hard plastic or rubber and are not worn on the body. Teething jewelry is made of beads of amber, wood, marble, or silicone. This jewelry may also be used by people with special needs to redirect chewing on body parts for clothing. These products can also cause injury to the mouth or an infection in the gums. The FDA has received reports of death and serious injuries to infants and children, including strangulations and … [Read more...]
FDA Takes Action Against OTC Teething Products That Contain Benzocaine
The FDA is warning consumers that over-the-counter (OTC) teething products that contain Benzocaine pose "a serious risk to infants and children." The agency announced that those products containing that pain reliever should no longer be marketed, and is asking companies to stop selling these products. If the companies do not comply, the FDA will initiate a regulatory action to remove those products from the market. The FDA is also asking that companies add warnings to all other benzocaine oral health products to describe some serious health risks. FDA Commissioner Scott Gottlieb said, "The FDA is committed to protecting the American public from products that pose serious safety risks, especially those with no demonstrated benefit. Because of the lack of efficacy for teething and the … [Read more...]
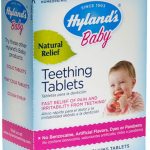
Hyland’s Baby Teething Tablets Recalled for Inconsistent Belladonna
Standard Homeopathic Company is recalling all lots of Hyland's Baby Teething Tablets and Hyland's Baby Nighttime Teething Tablets sold in retail stores because they have inconsistent amounts of belladonna alkaloids that may differ from the amount listed on the label. Belladonna represents a serious health hazard to children and its effects are unpredictable, according to the FDA. In fact, the Agency told the company, "There is no known safe dose or toxic dose of belladonna in children because of the many factors that affect it." These products are used to provide temporary relief of teething symptoms in children. And the recall includes all products that may be in stock at retail stores now. The Company stopped making and shipping the medicines in October 2016. The FDA has been … [Read more...]
FDA Investigating Homeopathic Teething Product Adverse Effects
The FDA is investigating more than 400 reports of adverse events associated with homeopathic teething products in the last six years, Lyndsay Meyer of the FDA's Office of Media Affairs told Food Poisoning Bulletin. In addition, they are "aware of reports of 10 deaths during that time period that reference homeopathic teething products, though the relationship of these deaths to the homeopathic teething products has not yet been determined and is currently under review," she stated. On September 9, 2016, the FDA received a comprehensive report of a recent adverse event of a child having a seizure associated with the use of a homeopathic teething product, triggering an investigation. Other adverse events reported to the government included "seizure, death, fever, shortness of breath, … [Read more...]
FDA Warns About Serious Effects of Homeopathic Teething Products
The FDA is warning consumers that using homeopathic teething tablets and gels can pose a health risk to infants and children. Consumers should stop using these products immediately and throw away any they may have in their homes. These products are distributed by CVS, Hyland's, and other stores, and are possibly sold in other retail stores and online. Adverse events in children who have been given these products are being investigated by the FDA. Some of the issues include seizures in infants and children. Dr. Janet Woodcock, director of the FDA's Center for Drug Evaluation and Research said in a statement, "we recommend parents and caregivers not give homeopathic teething tablets and gels to children and seek advice from their health care professional for safe alternatives." The … [Read more...]