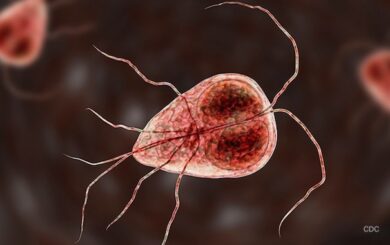
El Tapatito Giardia Outbreak in Kentucky Sickens Patients
An El Tapatito Giardia outbreak in Kentucky has sickened an unknown number of people, according to the Hopkins County Health Department. The tiny parasite Giardia duodenalis causes gastrointestinal illnesses and usually sickens people who swallow … [Read More...]















 Our editor, Linda Larsen, has written 52 cook books. She worked for the Pillsbury company in their test kitchens and for the Pillsbury Bake-Off. She holds a degree with High Distinction in Food Science from the University of Minnesota.
Our editor, Linda Larsen, has written 52 cook books. She worked for the Pillsbury company in their test kitchens and for the Pillsbury Bake-Off. She holds a degree with High Distinction in Food Science from the University of Minnesota.
recent+comments