The Food and Drug Administration (FDA) is warning five homeopathic product manufacturers for significant violations of current good manufacturing practice regulations (CGMP). Warning letters were sent to those companies. Four of the letters target companies who jointly produced a product labeled as homeopathic that was not sterile. The fifth outlines a company's failure to have a system in place that makes sure there is proper design, monitoring, and control of the manufacturing process. FDA Acting Commissioner Dr. Ned Sharpless said in a statement, "It’s our public health obligation to protect consumers from unsafe products. Our manufacturing requirements are designed to ensure the quality and safety of drugs distributed to American consumers, and the FDA expects companies to … [Read more...]
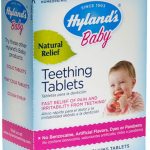
Hyland’s Baby Teething Tablets Recalled for Inconsistent Belladonna
Standard Homeopathic Company is recalling all lots of Hyland's Baby Teething Tablets and Hyland's Baby Nighttime Teething Tablets sold in retail stores because they have inconsistent amounts of belladonna alkaloids that may differ from the amount listed on the label. Belladonna represents a serious health hazard to children and its effects are unpredictable, according to the FDA. In fact, the Agency told the company, "There is no known safe dose or toxic dose of belladonna in children because of the many factors that affect it." These products are used to provide temporary relief of teething symptoms in children. And the recall includes all products that may be in stock at retail stores now. The Company stopped making and shipping the medicines in October 2016. The FDA has been … [Read more...]
CVS Homeopathic Products Recalled for Inconsistent Belladonna
Raritan Pharmaceuticals, a manufacturer for Homeolab USA, is recalling homeopathic products containing belladonna extract because there is potential for variation in the content of belladonna extract. The FDA tested some of the products and found varying levels of belladonna from what is declared on the label. Last month, the FDA warned consumers not to use homeopathic teething products for exactly this reason. Symptoms of belladonna toxicity include seizures, difficulty breathing, lethargy, excessive sleepiness, muscle weakness, skin flushing, constipation, and agitation. The recalled products include CVS Homeopathic Infants' Teething Tablet 135 tablets with UPC number 050428424162 and lot numbers 41116 and 43436. CVS has already taken a market action on this product as of … [Read more...]
FDA Warns About Serious Effects of Homeopathic Teething Products
The FDA is warning consumers that using homeopathic teething tablets and gels can pose a health risk to infants and children. Consumers should stop using these products immediately and throw away any they may have in their homes. These products are distributed by CVS, Hyland's, and other stores, and are possibly sold in other retail stores and online. Adverse events in children who have been given these products are being investigated by the FDA. Some of the issues include seizures in infants and children. Dr. Janet Woodcock, director of the FDA's Center for Drug Evaluation and Research said in a statement, "we recommend parents and caregivers not give homeopathic teething tablets and gels to children and seek advice from their health care professional for safe alternatives." The … [Read more...]