The ByHeart formula botulism outbreak case count has now sickened 37 infants in 17 states, according to the Centers for Disease Control and Prevention (CDC). That’s an increase of six new patients, six new hospitalizations, and two new states since the last update was issued on November 20, 2025. The new states are Massachusetts and Wisconsin.
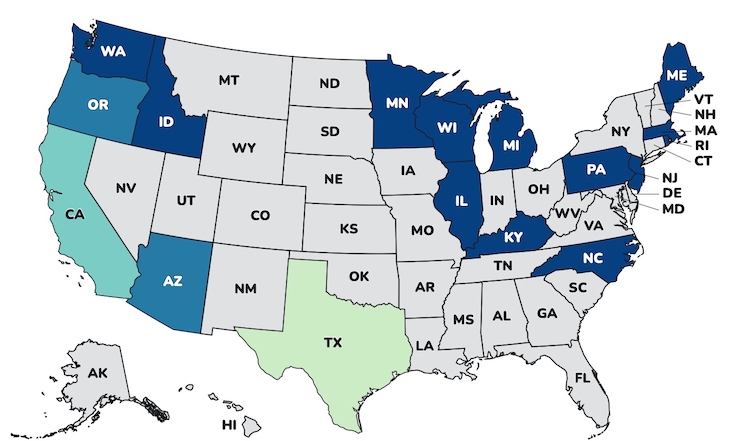
The case count by state is: Arizona (3), California (5), Idaho (1), Illinois (2), Kentucky (1), Massachusetts (1), Maine (1), Michigan (1) Minnesota (2), North Carolina (2), New Jersey (1), Oregon (3), Pennsylvania (1), Rhode Island (1), Texas (8), Washington (2), and Wisconsin (1). For 35 infants whose age was made available to public health officials, they range in age from 16 to 264 days. Illness onset dates range from August 9 to November 19, 2025 for 36 of these infants. All of the infants were hospitalized and treated with BabyBIG®, the FDA-approved treatment for this infection developed by the California Department of Health.
According to the FDA, all infants were fed ByHeart Whole Nutrition powdered infant formula. which was sold online and nationwide, including in Guam and Puerto Rico, before they got sick. All of that company’s formula has been recalled. There have been reports that the recalled formula is still for sale in several states, including at multiple Walmart, Target, and Kroger locations, and at one or more Sprouts Organic Market, Safeway, Acme, Jewel-Osco, Shaw’s, Star Market, Smith’s, King Sooper’s, Albertson’s, Whole Foods, Wegman’s, and Publix locations.
The FDA is releasing the Establishment Inspection Reports and FDA Form 483 reports that were conducted at three ByHeart facilities between 2022 and March 2025. ByHeart is the parent company of three Blendhouse Manufacturing facilities in Alelrton, Iowa; Portland, Oregon; and Reading, Pennsylvania. The Allerton location had GMP (Good Manufacturing Practice) deficiencies. The Portland facility was classified No Action Indicated, but the facility in Reading, inspected in January 2024, was classified Official Action Indicated. That plant has not been in operation since September 2023.
If you purchased ByHeart Whole Nutrition Infant Formula, do not feed it to your child. You can throw it away or return it to the place of purchase for a refund.
If you did feed it to your child, watch them carefully for the symptoms of infant botulism, which can take several weeks to appear. If they do seem to be getting sick, get them to an emergency room or a pediatrician as soon as possible. They may be a part of this ByHeart formula botulism outbreak.

If your child ha been sickened with a botulism food poisoning infection, please contact our experienced attorneys for help with a possible lawsuit at 1-888-377-8900 or text us at 612-261-0856. Our firm represents clients in lawsuits against grocery stores, restaurants, and food processors.




