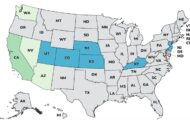
Similac Probiotic Tri-Blend is being recalled for serious adverse reactions to this product in preterm infants, according to the FDA. This notice was posted on the FDA's Enforcement Page, not the regular recall page. The FDA did issue a warning last month about problems with probiotics when administered to preemies, and a preterm infant died in early October 2023 after being given Evivo with MCT Oil probiotic in the hospital. The recalling firm is Abbott Laboratories. This product was distributed in these states: Alabama, Arkansas, Arizona, California, Colorado, Connecticut, Florida, Georgia, Iowa, Idaho, Illinois, Indiana, Kentucky, Louisiana, Massachusetts, Maryland, Maine, Michigan, Minnesota, Missouri, Mississippi, Montana, North Carolina, North Dakota, Nebraska, New Jersey, New … [Read more...]
Preterm Infant Dies After Receiving Evivo with MCT Oil Probiotic
A preterm infant has died after receiving Evivo with MCT Oil probiotic in the hospital. That dietary supplement, produced by Infinant Health, is formulated to contain the live bacterium Bifidobacterium longum subsp. infantis, according to the FDA. The infant developed sepsis caused by that bacterium. The FDA is investigating this death. Genomic sequencing data found that the bacteria that caused sepsis in the infant was a genetic match to the bacteria in the probiotic. The FDA is now warning that preterm infants who are given these dietary supplements are "at risk of invasive, potentially fatal disease caused by the bacteria or fungi contained in probiotics." Medical literature reports state that microorganisms in probiotics have caused bacteremia or fungemia, sometimes with severe … [Read more...]
GoHealthy Probiotics Recalled for Potential Microbial Growth
GoHealthy Probiotics and Ozona Probiotics are being recalled because they may have the potential to become contaminated with microbial growth. This can cause illness, especially since two of the products are intended for infants, toddlers, and kids. No illnesses have been reported to the company to date in connection with the consumption of these products. The recalling firm is Ozona Organics LLC of Ozona, Texas. The recalled products are 4 ounce and 16 ounce bottles of Ozona Probiotics for Digestive Health (intended for human use), also labeled as GoHealthy Probiotics for Infants, Toddlers and Kids in 2 ounce bottles, and GoHealthy Probiotics for Infants, Kids, Men and Women in 4 ounce bottles. The products have high water activity that provides the potential for microbial growth, … [Read more...]
Liviaone Liquid Probiotics Recalled For Pseudomonas aeruginosa
Liviaone Liquid Probiotics, Topical Spray, Nasal Probiotics, and BioLifePet Liquid Probiotics are being recalled because they could be contaminated with Pseudomonas aeruginosa, a microorganisms that could cause life-threatening infections in immunocompromised people and animals. These types of infections usually occur in hospital settings. Six of these products are dietary supplements for people, and two are for animals, both cats and dogs. No illnesses have been reported to the company to date in connection with this problem. The recalling firm is Livia Global, Inc. of Visalia, California. The recalled Liviaone Liquid Probiotics are available in nasal sprays and as liquid probiotics, the type to be ingested. Two lots of the human products are recalled: the ingested products have … [Read more...]