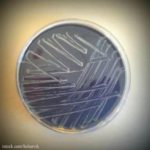
The FDA warned ByHeart about insanitary conditions in their facility more than two years ago during the worry about Cronobacter in infant formula. A warning letter was sent on August 30, 2023. The letter states, "During our inspection at the (b)(4) facility, FDA investigators found significant violations of Title 21, Code of Federal Regulations, Part 106 (21 C.F.R. Part 106), Infant Formula Requirements Pertaining to Current Good Manufacturing Practice, Quality Control Procedures, Quality Factors, Records and Reports, and Notifications (“the Infant Formula Rule”)." This information is interesting because powdered ByHeart Whole Nutrition Infant Formula is linked to a botulism outbreak that has sickened 15 infants in 12 states. The California Department of Health conducted testing on … [Read more...]
Warning Letter to FluxxLab For Unapproved COVID Treatments
The FDA has sent a warning letter to FluxxLab LLC because th company is allegedly selling COVID-19 treatments that have no proof of efficacy. The letter states, "The FDA has observed that your website offers the Covid-19 Immune Support Tincture and the CBDA+CBD Oil Tincture products for sale in the United States and that these products are intended to mitigate, prevent, treat, diagnose, or cure COVID-19 in people. Based on our review, these products are unapproved new drugs sold in violation of section 505(a) of the Federal Food, Drug, and Cosmetic Act (FD&C Act), 21 U.S.C. § 355(a). "Furthermore, these products are misbranded drugs under section 502 of the FD&C Act, 21 U.S.C. § 352. The introduction or delivery for introduction of these products into interstate commerce is … [Read more...]
Jule’s Foods FDA Warning Letter Details Salmonella Contamination
A Jule's Foods FDA warning letter details the Salmonella contamination found at that company's facility, after inspections were launched in relation to a multistate outbreak that sickened at least 20 people in four states and hospitalized five. The outbreak was declared over in early July 2021. The facility is located at 2790 Loker Avenue West in Carlsbad, California. The FDA inspected the facility from April 21 to May 24, 2021. The outbreak included six Salmonella serotypes. Four of those serotypes were found in an environmental swab taken from the company's vegan cashew brie, the vegan cashew brie varieties, and/or the incoming cashew pieces used to make these products. The CDC and FDA determined, based on epidemiologic and laboratory evidence, that the RTE vegan cashew brie … [Read more...]
FDA Warns Two Companies Selling OTC CBD Products For Pain Relief
The FDA has issued warning letters to two companies selling OTC CBD products for pain relief. The items are labeled as containing cannabidiol (CBD) in ways that violate the Federal Food, Drug, and Cosmetic Act (FD&C Act). The letters address the illegal marketing of unapproved drugs labeled as containing CBD. The FDA has not approved any over-the-counter (OTC) drugs containing CBD, and these products do not meet the requirements to be legally marketed without an approved new drug application. CBD has known pharmacological effects on people, with demonstrated risks, so it can't be legally marketed as an inactive ingredient in OTC drug products that are not approved by the FDA. The letters also cite substandard manufacturing practices, including the failure to comply with … [Read more...]
FDA Warns Mercola Over Products Claiming to Treat and Cure COVID-19
The FDA has sent a warning letter to Mercola.com about their products being marketed as diagnostic tools, treatment options, and cures for COVID-19. The letter, dated February 18, 2021, addresses "Unapproved and Misbranded Products Related to Coronavirus Disease 2019." The company's websites and social media accounts promote these products which are considered unapproved new drugs sold in violation of the FDA's Food, Drug and Cosmetic Act. The FDA is taking urgent measures to protect consumers that, without approval or authorization by that agency, claim to "mitigate, prevent, treat, diagnose, or cure COVID-19 in people." The government states that Mercola must take immediate action to cease the sale of these unapproved and unauthorized products. Some of the claims on the … [Read more...]
FDA Warning Letter to Dole Fresh Vegetables Mislabeled Tuscan Herb Salad
The FDA has sent a warning letter dated September 25, 2020 to Dole Fresh Vegetables at 2959 Salinas Highway in Monterey, California, after failure to comply with preventive controls of thee Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Human Food regulation. The product, HEB Tuscan Herb Chopped Salad Kit, was recalled in March 2020 for undeclared peanuts, wheat, soy, and tree nuts, four of the major food allergens. The FDA states that the company said that the mislabeling occurred when the incorrect masterpack kit, which is the kit containing the dressing and other toppings, was "unintentionally used during the production of the salad." The company also stated that the firm "did not have a robust enough verification process for product … [Read more...]
FDA Warns Maker of Chlorine Dioxide Products Used for COVID-19
The FDA has issued a warning letter to Genesis II Church of Health and Healing that markets fraudulent and dangerous chlorine dioxide products for the prevention and treatment of COVID-19. The company makes a product called "Miracle Mineral Solution." The FDA has warned consumers not to purchase or drink chlorine dioxide products that are sold as medical treatments online. There is no scientific evidence supporting this compound's safety or effectiveness. These products also pose significant risks to patient health. FDA Commissioner Dr. Stephen M. Hahn said in a statement, "Despite previous warnings, the FDA is concerned that we are still seeing chlorine dioxide products being sold with misleading claims that they are safe and effective for the treatment of diseases, now including … [Read more...]