Botanic Choice Prune & Senna Softgels, a dietary supplement, is being recalled because it may contain peanuts, one of the nine major food allergens, that is not declared on the label. Anyone who is allergic to peanuts could have a serious or life-threatening allergic reaction if they consume this product. No illnesses have been reported to the company to date in connection with the consumption of this product. The recalling firm is Indiana Botanic Gardens of Indiana. This dietary supplement was sold nationally in the company's retail stores and also through online and mail orders. The recalled product, Indiana Botanic Choice Prune & Senna Softgels, is packaged in a 30 count white plastic bottle with an orange and green label. The lot code that is printed on the label is … [Read more...]
Airborne Gummies Recalled For Possible Injury Hazard
Airborne Gummies are being recalled for a possible injury hazard, according to the Consumer Product Safety Commission. Pressure can build up in these dietary supplement bottles and the cap and seal can pop off with force, posing an injury hazard. There have been 70 reports of the ap or seal popping off the bottles, including 18 reports of minor injuries and one report of an eye injury that required medical attention. The recalling firm is RB Health (US) LLC (Reckitt"), of Parsippany, New Jersey. When opened for the first time, the pressure could cause the cap and seal to pop off with force. The recalled Airborne Gummies were only sold in two sizes - 63 count and 75 count. The UPC numbers are on the product label and the lot codes and expiration dates are on the bottom of the bottle. … [Read more...]
BUBS Naturals Fountain of Youth Formula Recalled For Milk
BUBS Naturals Fountain of Youth Formula with two lot numbers is being recalled because it may contain milk, one of the major food allergens, that is not declared on the label. That means that anyone who is allergic to milk, or who is lactose intolerant, could have a serious reaction if they consume this product. No reports of illness or allergic reactions have been received by the company to date in connection with the consumption of this item. The recalling firm is BUBS Naturals of Encinitas, California. The dietary supplement product was distributed through BUBS Naturals online store, through Amazon, and through some grocery stores. The recalled product is BUBS Naturals Fountain of Youth Formula packaged in 10.16 ounce containers. The two lot numbers that are recalled are 04230401 … [Read more...]
Liviaone Liquid Probiotics Recalled For Pseudomonas aeruginosa
Liviaone Liquid Probiotics, Topical Spray, Nasal Probiotics, and BioLifePet Liquid Probiotics are being recalled because they could be contaminated with Pseudomonas aeruginosa, a microorganisms that could cause life-threatening infections in immunocompromised people and animals. These types of infections usually occur in hospital settings. Six of these products are dietary supplements for people, and two are for animals, both cats and dogs. No illnesses have been reported to the company to date in connection with this problem. The recalling firm is Livia Global, Inc. of Visalia, California. The recalled Liviaone Liquid Probiotics are available in nasal sprays and as liquid probiotics, the type to be ingested. Two lots of the human products are recalled: the ingested products have … [Read more...]
Mountain Meadow Herbs Candida Flush Recalled For Exploding Bottle Risk
Mountain Meadow Herbs Candida Flush dietary supplement is being recalled because the contents may explode out of the bottle when the container is opened, posing an injury hazard to hands and eyes. No illnesses or serious injuries have been reported to the company to date in connection with this problem. The recalling company is Mountain Meadow Herbs of Somers, Montana. Fifty four bottles of this product are being recalled. Some of the bottles from one particular lot became over pressurized during storage. The product may "forcefully expel air" as well as portions of capsules and powder when opened. The recalled product was sold to retailers in Indiana, Minnesota, Montana, New York, and in Tennessee, and also in Ontario, Canada. The item was also sold directly to consumers in … [Read more...]
Nutracap Dietary Supplements Recalled For Undeclared Allergens
Nutracap dietary supplements, including the brands Boba Origin, Etedream, RAW, Steel, and Vital Force, are being recalled for the allergens wheat, milk, soy, and coconut that are not declared on the label. Anyone who is allergic to those ingredients, including people who have lactose intolerance or celiac disease, could have a serious reaction if they eat them. No illnesses or allergic reactions have been reported by the company to date. These Nutracap dietary supplements were shipped to Alabama, California, Florida, and Texas from June 4, 2020 though October 1, 2021. During an FDA inspection on November 4, 2021, the firm was notified that their labels did not disclose the presence of these allergens on some of their products. You can see pictures of the recalled products at the FDA … [Read more...]
ITS Liquid Probiotic for Infants Recalled For Bacterial Contamination
ITS Liquid Probiotic for Infants is being recalled for possible contamination with Pseudomonas aeruginosa. The recall notice states, "The only product complaint the company has received with respect to the affected product lots was one report of temporary diarrhea in an older infant after consuming the product, which the company does not believe was related to the presence of the microorganism." The recalling company is MaryRuth's, which manufacturers vitamins, minerals, and supplements. Pseudomonas aeruginosa can cause infection in immunocompromised people or, rarely, in very young infants. The infant's immature gut may not be able to prevent the pathogen from getting into th bloodstream, which can cause serious illness. The recall is for ITS Liquid Probiotic for Infants that is … [Read more...]
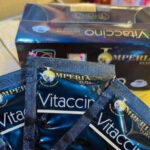
Imperia Elita Vitaccino Coffee Recalled For Undeclared Drugs
Dash Exclusive is voluntarily recalling all lots of Imperia Elita Vitaccino Coffee because it contains sibutramine and fluoxetine, two drugs that are not declared on the label. This product is a weight loss and anxiety dietary supplement. FDA analysis found the presence of these compounds in the product. The company has not received any reports of adverse reactions related to the use of this recalled product. Sibutramine was an FDA-approved drug that was used as an appetite suppressant. It was withdrawn from the marketplace because of safety issues, including stroke, heart failure, and serious health risks, especially for people who have underlying heart disease. Sibutramine can significantly increase blood pressure and pulse rate. Fluxoetine is an FDA-aprpved drug that is used … [Read more...]
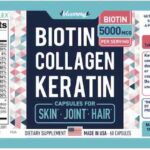
Blommy Biotin Collagen Keratin Recalled For Undeclared Fish
Blommy, Inc. is recalling Blommy Biotin Collagen Keratin Capsules for fish one of the major food allergens, that is not declared on the label. Anyone who is allergic to fish could have a serious allergic reaction if they consume this product. No allergic reactions or illnesses have been reported to date in connection with the consumption of this product. The recalled item is Blommy Biotin Collagen Keratin Capsules for Skin, Joint, and Hair. There are 60 capsules in each bottle 2.08 ounce bottle. The capsule details are 00 clear vegetable capsule. The manufacture date is 04/2021, and the best by date is April 2023 (04/2023). This product was sold through Amazon and the company's online retail website. A total of 13,688 units were sold. Blommy, Inc. was told about this allergen by … [Read more...]
vitafusion Gummy Vitamins Recalled For Foreign Material
Church & Dwight Company is voluntarily recalling vitaFusion Gummy Vitamins dietary supplements because they may contain foreign material, more specifically metallic mesh material. This poses a choking and injury hazard. In severe cases, consuming a metallic mesh material could damage the digestive tract. There have been no reports of consumer illness or injury received by the company to date in relation to the consumption of these products. The recall was prompted by two consumer reports. The recalled vitamins were manufactured during a four day period between October 29, 2020 and November 3, 2020. They were sold to in-store and e-commerce retailers from November 13, 2020 to April 9, 2021. The recalled products include vitafusion Kids Melatonin in 50 count packages, with UPC … [Read more...]