The FDA says that some Tejocote root supplements are adulterated with toxic yellow oleander, which is a poisonous plant that is native to Mexico and Central America. The tested products are labeled Tejocote but are actually toxic yellow oleander. The supplements are usually sold online through third-party platforms. Ingestion of yellow oleander can cause serious health problems that can be fatal. The plant can cause neurologic, gastrointestinal, and cardiovascular adverse health effects. Symptoms include nausea, vomiting, dizziness, diarrhea, abdominal pain, cardiac changes, dysrhythmia, and more. In September 2023, the CDC published a report of several Tejocote root products that contained yellow oleander. The FDA initiated an investigation to sample and test more products. More … [Read more...]
FDA Warns Against Red’s Best Illegally Harvested Chopped Clams
The FDA is warning restaurants not to serve Red's Best illegally harvested chopped clams for food safety reasons. Restaurants and food retailers in Connecticut, Massachusetts, New York, and Rhode Island may have purchased the clams. It is possible that the chopped clams may have been distributed to other states as well. The FDA is waiting for more information on the distribution of these chopped clams. The clams are labeled as #331 and #333, with shuck dates of 23/331 and 23/333. They were illegally harvested from prohibited waters in Massachusetts on November 25, 2023 and November 26, 2023. Clams that are illegally harvested may be contaminated with human pathogens, toxic compounds, or poisonous substances and can cause illness if eaten, even if they have been cooked. Clams are … [Read more...]
FDA’s Annual Warning About Consuming Raw Flour
It's time for the FDA's annual warning about consuming raw flour, since the holiday season is a traditional time for a lot of baking. There have been many outbreaks linked to raw flour and products made with raw flour, such the Salmonella outbreak linked to recalled Gold Medal flour earlier this year, and the E coli outbreak linked to cake mix two years ago. Because it is dry, flour seems so innocuous. But pathogens such as Salmonella and E. coli can easily survive in low water foods. And flour is a raw agricultural product made from raw grains, which means that it can be contaminated with pathogens in the field, during harvest or transport, or during processing. The only thing that makes flour safe to eat is heat. Do not eat any uncooked cookie dough or batter. And do not make … [Read more...]
FDA Warns Against Neptune’s Fix Because of Serious Health Risks
The FDA is warning consumers not to buy or use Neptune's Fix, a product that is marketed for pain relief, to improve brain function, and treat anxiety, depression, and opioid use disorder. The government has received reports of serious complications after the use of this product, including seizures and loss of consciousness. The product contains tianeptine, which is a substance that is not approved by the FDA for any medical use. Tianeptine is considered an alternate to fentanyl and oxycodone. While some companies in this country are planning to seek FDA approval for this drug, that has not happened. According to Consumer Reports, tianeptine-related calls to poison controls centers have "skyrocketed" since 2015. At least four people in the United States have died after using the … [Read more...]
FDA Plans to Ban Brominated Vegetable Oil in Food
The FDA plans to ban the use of brominated vegetable oil (BVO) in food, after studies conducted with the National Institutes of Health found the product is potentially dangerous for human consumption. Brominated vegetable oil is a mixture of brominated triglycerides that are made by combining bromine with unsaturated vegetable oils. BVO is authorized for use in small amounts to keep citrus flavoring from separating and floating to the top of some beverages. The FDA took BVO off the Generally Recognized as Safe (GRAS) list in 1970 and began overseeing its use under food additive regulations. Many beverage makers have reformulated their products to replace BVO. Now, few beverages available in the United States contain BVO. Scientists found that the thyroid is a target organ of … [Read more...]
FDA Issues Updated Compliance For Infant Formula
The FDA has issued updated compliance for infant formula after the disastrous shutdown of Abbott Nutrition for cronobacter contamination in 2022 that caused severe shortages. The press release states that, "This effort is part of the FDA's ongoing commitment to strengthen the safety, resiliency, and oversight of the infant formula industry." The FDA released its internal evaluation of the response in 2022. It recommended that the agency review and update its compliance program to make sure it reflected the latest science on Cronobacter, and offered consistency and clarify for manufacturers on inspection and compliance activities. The FDA has published its updated compliance program, which it says builds on the lessons learned over the last several years. Salmonella and … [Read more...]
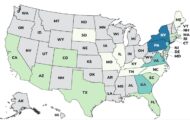
California Bans Red 3, Brominated Vegetable Oil, Other Additives
California bans Red 3, brominated vegetable oil, potassium bromate, and propylparaben from being added to food because of possible adverse health effects. The governor signed the legislation on October 7, 2023. Industry has until 2027 to comply with this new law. The synthetic dye, Red 3, is made from petroleum. The law, Assembly Bill 418, makes California the first state in the country to ban the use of these additives in packaged foods. The FDA says they are safe to consume. Center for Science in the Public Interest (CSPI) states that the FDA learned Red 3 was a carcinogen in the 1980s and declared it as a carcinogen in 1990. This 2012 article from The National Library of Medicine states that Red 3 causes cancer in animals. The FDA has eliminated the use of this food dye from … [Read more...]