Hy Vee potato salad is being withdrawn from stores for possible contamination. A presumptive positive microbial test result was found on the line that processes the potatoes. The press release did not state which pathogen may be the cause of the positive test. All potato salad varieties that the company sells are included in this recall. The final test results are not expected for about a week, but out of an abundance of caution the company is withdrawing the salad. The recalled product includes all varieties and sizes of Hy-Vee Potato Salad and Mealtime potato salad sold from the grab-and-go refrigerated cases and/or deli service cases in all Hy Vee, Hy-Vee Drugstore, and Dollar Fresh convenience stores. These stores are located in Illinois, Iowa, Kansas, Minnesota, Missouri, … [Read more...]
One Quarter of Participants Contaminated Salad With Raw Chicken
In an NC State University study, one quarter of participants contaminated salad with raw chicken, highlighting the risks of not understanding hand washing, cleaning and sanitizing the kitchen, and washing poultry. The study was conducted to assess the impact of washing poultry on kitchen contamination, but found that participants who did not wash poultry still contaminated salad. The study was published in the International Association for Food Production. Washing raw poultry is not recommended because the researchers thought that water from rinsing the chicken would splash and spread around the kitchen. This practice can also contaminate the kitchen sink, other foods, and other surfaces, which increase the risk of foodborne illness. Ellen Shumaker, corresponding author of the … [Read more...]
Senna Syrup Recalled For Possible Microbial Contamination
Senna Syrup is being recalled for possible microbial contamination. This product is a natural vegetable laxative. The contamination could cause serious or life-threatening illnesses among certain populations, more specifically the elderly, anyone with a weakened immune system, or patients at higher risk of developing life-threatening inflammation of the heart. No reports of illness have been received by the company to date. The recalling firm is Lohxa LLC of Worcester, Massachusetts. The recalled product is Senna Syrup that is packaged into 5 mL unit-dose cups. The product is sold in case of 20 cartons packaged with 24 units each. The NDC is 50268-731-24. The recalled product 8.8 mg/5mL lot number is AM1115S, and the expiration date stamped on the label is 01/2023. The syrup was … [Read more...]
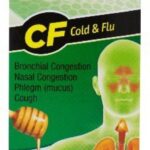
Rompe Pecho Cold Treatment Recall For Possible Contamination Updated
Rompe Pecho cold treatments and flu treatments are being recalled because they may have microbial contamination. These lots were distributed in 2019, although the products all expire next year. This is an expansion of a 2019 recall. No reports of illness have been received by the company to date in connection with the consumption of these products. The recalling firm is Efficient Laboratories, Inc. of Miami, Florida. The original recall was for one lot each of Rompe Pecho EX, Rompe Pecho CF, and Rompe Pecho MAXliquid. The recall notice states that the microbes could cause vomiting and diarrhea in "rare circumstances." This update adds an additional 12 lots of these products to the recall total. Each item is packaged in a box containing a bottle of the liquid. The recalled … [Read more...]
Liviaone Liquid Probiotics Recalled For Pseudomonas aeruginosa
Liviaone Liquid Probiotics, Topical Spray, Nasal Probiotics, and BioLifePet Liquid Probiotics are being recalled because they could be contaminated with Pseudomonas aeruginosa, a microorganisms that could cause life-threatening infections in immunocompromised people and animals. These types of infections usually occur in hospital settings. Six of these products are dietary supplements for people, and two are for animals, both cats and dogs. No illnesses have been reported to the company to date in connection with this problem. The recalling firm is Livia Global, Inc. of Visalia, California. The recalled Liviaone Liquid Probiotics are available in nasal sprays and as liquid probiotics, the type to be ingested. Two lots of the human products are recalled: the ingested products have … [Read more...]
ITS Liquid Probiotic for Infants Recalled For Bacterial Contamination
ITS Liquid Probiotic for Infants is being recalled for possible contamination with Pseudomonas aeruginosa. The recall notice states, "The only product complaint the company has received with respect to the affected product lots was one report of temporary diarrhea in an older infant after consuming the product, which the company does not believe was related to the presence of the microorganism." The recalling company is MaryRuth's, which manufacturers vitamins, minerals, and supplements. Pseudomonas aeruginosa can cause infection in immunocompromised people or, rarely, in very young infants. The infant's immature gut may not be able to prevent the pathogen from getting into th bloodstream, which can cause serious illness. The recall is for ITS Liquid Probiotic for Infants that is … [Read more...]
Durisan Antimicrobial Hand Sanitizer Recalled For Microbial Contamination
Durisan Antimicrobial Hand Sanitizer in several package sizes is being voluntarily recalled for possible microbial contamination. The recall was issued because of out of specification results of bacterial counts for Burkholderia cepacia complex and Ralstonia pickettii. The issue was discovered during a routine audit that was focused on production scale-up during the height of the coronavirus pandemic. No reports of adverse reactions or consumer complaints have been received by the company to date. The Durisan Antimicrobial Hand Sanitizer was packaged in sizes that ranged from 18 mL credit cards, to bottles that were sized 118 mL, 236 mL, 300 mL, and 500 mL. It was also available in 1000 mL wall mounted dispenser refills. You can see pictures of recalled product labels at the FDA web … [Read more...]
Maison Terre Goldenseal Root Powder Recalled; One Infant Has Died
Maison Terre of Little Rock, Arkansas is recalling all lots of Maison Terre Goldenseal Root Powder for bacterial contamination. FDA laboratory analysis of product samples found the product is contaminated with Enterobacter cloacae, Cronobacter sakazakii, and Cronobacter dublinensis, among others. This product was purchased from Starwest Botanicals of Sacramento, California, and repackaged to sell to consumers. The recall notice states, "The use of contaminated product in otherwise healthy patients can result in infections necessitating antimicrobial and potentially surgical treatment. In individuals with weak immune systems and infants, the use of the product can result in death. Maison Terre has received a report of one infant death associated with use of this product on the … [Read more...]
Can Spinach Be Contaminated With Pathogens At Germination?
We have all heard about the several deadly E. coli O157:H7 outbreaks linked to romaine lettuce that sickened hundreds of people last year in the United States. That contamination apparently occurred while the greens were growing in the field. But can leafy greens such as spinach be contaminated with pathogens during germination? Unfortunately, leafy greens are a main source of foodborne illness, because they are easily contaminated in the field and typically aren't cooked before serving. But that contamination was on the surface of the plant, not internalized into the leaves themselves. Now researchers have looks at the possibility that spinach can be contaminated at germination. Even worse, this contamination can be internalized in the leaf, which means that no amount of … [Read more...]
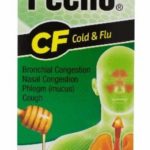
Rompe Pecho Products Recalled For Microbial Contamination
Efficient Laboratories is voluntarily recalling one lot each of Rompe Pecho EX, CF, and MAX liquid. These products are used to treat symptoms of the flu and the common cold. The three lots have been found to contain microbial contamination. The recall notice did not state what type of microbe may have contaminated these products, but it did state that "In rare circumstances, consumption of Rompe Pecho from these lots could result in vomiting and diarrhea. Efficient Laboratories has not received any reports of adverse events to date." Each product is packaged in a box containing one bottle of the liquid product. The product lots are: Rompe Pecho EX lot 19F332, with expiration date June 2022, Rompe Pecho CF lot 19H359, with expiration date of August 2022, and Rompe Pecho MAX lot 19B42, … [Read more...]