Similac Cronobacter recall support is being offered by the USDA so WIC participants can exchange recalled powdered infant formula. Certain lots of Similac, EleCare, and Alimentum formula are being recalled for possible Cronobacter and Salmonella contamination after four infants got sick. The formula is offered through the Special Supplemental Nutrition Program for Women, Infants, and Children. The USDA Food and Nutrition Service is "strongly encouraging" agencies in affected states, territories, and tribal nations to take immediate action to make sure that WIC participants can exchange recalled baby formula and can use WIC credits to purchase product that has not been recalled. The agencies can request waivers of certain WIC regulations for maximum flexibility. The waivers that … [Read more...]
Minnesota Cronobacter Case Spurred Federal Investigation
In St. Louis County, MN, which encompasses the central and eastern portions of the Iron Range as it stretches from Duluth to the Canadian border, the leaves were reaching peak color when one of its newest and tiniest residents fell gravely ill. It was September 2021. The baby had developed a Cronobacter infection and would spend 22 days in the hospital successfully fighting it off. But because Minnesota is the only state in the nation that requires reporting of Cronobacter, which is known to cause severe, often fatal, infections in infants, that was not the end of the story. Reportable or notifiable diseases are considered to be of such great public health importance that when they are diagnosed they must be reported to state health officials who, in turn, report them to the … [Read more...]
The Long, Sad History of Cronobacter and Baby Formula
If you're lucky, the first time you ever heard the word "Cronobacter" was on a news segment last week. The foodborne pathogen with a name that sounds like a sci-fi villain doesn't make headlines often. And that's good. Because Cronobacter is only known for one thing - causing severe, often fatal, illness in newborn babies. And its main vehicle of transmission is powdered infant formula. Last week, federal health officials announced that they are investigating a possible link between Similac, EleCare, and Alimentum powdered baby formulas and the illnesses of four infants who consumed them. Three of the infants, in Minnesota, Ohio, and Texas, developed Cronobacter infections. One of them, another infant in Texas, developed a Salmonella infection. All four of the babies were … [Read more...]
What is Cronobacter Sakazakii? And Why is it a Danger in Infant Formula?
What is Cronobacter sakazakii? And why is it a danger in powdered infant formula? This pathogen is not well known but can cause serious illness and death in infants, especially babies who were premature, low birth weight, or have other health conditions or compromised immune systems. It is one of the costliest foodborne pathogens because of loss of life and serious health problems that can persist even when a patient recovers. The fatality rate in infants, even older infants, ranges from 40 to 80%. The cost of each case is estimated at $1,000,000, according to a study published in Front Microbiol. Despite the severity and high mortality rate of these infections, Cronobacter illnesses are not reportable conditions in the United States except in one state: Minnesota. That means these … [Read more...]
Recalled Baby Formula Still on Store Shelves
A major baby formula recall for Similac, EleCare, and Alimentum was issued five days ago, so why are these products still on store shelves? On Friday, February 17, Abbott Nutrition issued a baby formula recall for Similac, Alimentum, and EleCare after four illnesses were reported among infants who consumed them. The Cronobacter and Salmonella illnesses were reported from three states, MN (1), OH (1), and TX (2). All four babies were hospitalized and Cronobacter may have contributed to a death in one case, according to the U.S. Food and Drug Administration (FDA). After Food Poisoning Bulletin received reports that consumers were still finding recalled baby formula on store shelves, we went out this morning and took a look ourselves. Five days after the recall, we found recalled … [Read more...]
FDA Boosts Outreach Amid Baby Formula Cronobacter Concerns
As concerned parents scramble for information in the wake of a Cronobacter baby formula recall, the FDA is amplifying the methods consumers can use to ask questions or submit complaints. On Friday, the U.S. Food and Drug Administration (FDA) issued a consumer advisory about Similac, EleCare, and Alimentum baby formulas made at Abbott Nutrition’s Sturgis, MI facility. The advisory was prompted by three reports of Cronobacter sakazakii infections and one report of a Salmonella Newport infection in infants who had consumed these products. All four infants were hospitalized and Cronobacter may have contributed to a death in one case, according to the FDA. “As this is a product used as the sole source of nutrition for many of our nation’s newborns and infants, the FDA is deeply concerned … [Read more...]
More Information About Similac Alimentum EleCare Formula Recall
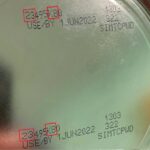
More information about the Similac Alimentum EleCare formula recall has been provided by DC Health, including lookup information for parents that the FDA has also supplied. The formula is associated with three Cronobacter illnesses and one Salmonella illness in infants who live in Minnesota, Texas, and Ohio. All four babies were hospitalized, and one infant died: the FDA states that "Cronobacter may have contributed to a death in one case." The more detailed information in the DC Health release is that parents should not use "Similac Advance, Similac Sensitive, Similac Total Comfort, Similac for Spit Up, Alimentum, EleCare Infant, and EleCare Jr. powdered formula," as long as the first two digits of the code are 22 through 37, the code on the container contains K8, SH, or Z2, and … [Read more...]
Infant Formula Cronobacter: What You Need to Know
Infant formula cronobacter is an issue that is not well known. Four infants are sick, three with Cronobacter infections and one with a Salmonella infection, according to the FDA, after being fed powdered infant formula. What is cronobacter and why is it an issue in this product? And how can you protect your child? The illnesses were announced on February 17, 2022 by the FDA. The infants live in Minnesota, Ohio, and Texas. The babies allegedly got sick from September 6, 2021 through January 11, 2022. One death has been reported but has not been confirmed to be "solely attributable" to Cronobacter infection, according to the notice. The FDA is advising parents not to use Similac, Alimentum, or EleCare powdered infant formulas, if the first two digits of the codes on the can are 22 … [Read more...]
Similac, Alimentum and EleCare Baby Formula Recall
Abbott Nutrition has issued a baby formula recall for Similac, Alimentum, and EleCare after four illnesses were reported among infants who consumed them. The Cronobacter and Salmonella illnesses were reported from three states, MN (1), OH (1), TX (2). between September 6, 2021, and December 18, 2021 Three of the infants developed Cronobacter sakazakii infections and one of them contracted a Salmonella Newport infection. All were hospitalized and Cronobacter may have contributed to a death in one case, according to the U.S. Food and Drug Administration (FDA). Cronobacter illnesses are rare but can be deadly for newborns because they can cause sepsis and meningitis, according to the Centers for Disease Control and Prevention (CDC). For infants, symptoms of sepsis or meningitis include … [Read more...]
Similac, EleCare, Alimentum Investigated by FDA for Possible Link to Cronobacter, Salmonella Outbreak
Federal health officials are investigating a possible link between Similac, EleCare, and Alimentum powdered instant baby formulas and a Cronobacter and Salmonella outbreak. All three products were made at Abbott Nutrition’s facility in Sturgis, MI. The FDA is advising consumers to avoid buying or using these powdered infant formula products which have the following identification on the bottom of the can. the first two digits of the code are 22 through 37 and the code on the container contains K8, SH, or Z2, and the expiration date is 4-1-2022 (APR 2022) or later. The FDA and the Centers for Disease Control and Prevention (CDC) are investigating four illnesses among infants who consumed products made at Abbott Nutrition’s Sturgis, MI facility. The illnesses were … [Read more...]