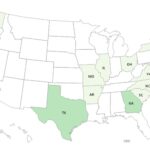
A Jif peanut butter Salmonella Senftenberg outbreak has sickened at least 14 people in 12 states. Two people have been hospitalized. The peanut butter was produced at the J.M. Smucker Company facility in Lexington, Kentucky. The patient case count by state is: Arkansas (1), Georgia (2), Illinois (1), Massachusetts (1), Missouri (1), Ohio (1), North Carolina (1), New York (1), South Carolina (1), Texas (2), Virginia (1), and Washington (1). The last illness onset date was May 1, 2022. We don't yet know the patient age range or first illness onset date. This outbreak could grow since it takes weeks from when a person first feels ill to when samples are tested and reported to the CDC. The distribution of these products was nationwide. They have been confirmed for the states listed … [Read more...]
St. Joseph’s Catholic Church Salmonella Outbreak in Amarillo, Texas
A St. Joseph's Catholic Church Salmonella outbreak has sickened a number of people, according to the City of Amarillo Public Health Department (APHD) and the City of Amarillo Environmental Health Department. The notice did not specify how many people are allegedly sickened in this outbreak. There is also no word on any hospitalizations, illness onset dates, or the patient age range. The outbreak is associated with an enchilada meal that was served at that church on Sunday, March 27, 2022. Anyone who has food items leftover from that event should discard them immediately. Anyone who is experiencing the symptoms of a Salmonella infection should see their doctor. And if you are sick, do not return to work, school, or daycare until you have been symptom-free for 24 hours. The notice … [Read more...]
Abbott Nutrition Form 483 Released by FDA in Cronobacter Illness Investigation
Abbott Nutrition Form 483 has been released by the FDA in the investigation of four Cronobacter illnesses linked to recalled Similac powdered infant formula. The three forms were released after inspections were conducted by the FDA at Abbott's facility in Sturgis, Michigan. All of the sick infants, who lived in Minnesota, Texas, and Ohio, are reported to have consumed powdered infant formula that was produced at that facility. The inspections were conducted on September 16 - 24, 2019, Sepatember 20 - 24, 2021, and January 31 - March 18, 2022. The notice states, "The inspectional observations in these Form 483s do not constitute final FDA determinations of whether any condition was or is in violation of the Federal Food, Drug, and Cosmetic Act or any of its implementing regulations. … [Read more...]
FDA Updates the Recalled Cronobacter Infant Formula Product List
The FDA has updated the recalled Cronobacter infant formula list with a full list of recalled products. In addition, they say that metabolic and other medical specialty infant formulas were produced at the same plant which made the recalled Similac PM 60/40, but those products were not recalled "because the FDA has determined that the risk of not having these specialty products available could significantly worsen underlying medical conditions. For many of these patients, the risk of life-threatening adverse events from restricted access to these critically needed products is likely greater than the risk from consuming products that have been produced at the facility." So far, at least four infants, who lived in Minnesota, Texas, and Ohio, have been diagnosed with Cronobacter … [Read more...]
FDA Removed a Salmonella Illness From Powdered Infant Formula Count
The FDA removed a Salmonella illness from the powdered infant formula case count, accord to an update issued for that agency's CORE Outbreak Investigation Table. The Table itself does not contain this information; it was sent in an email. Salmonella has been removed from the Pathogen column in that outbreak investigation. There are still four infants in the case count linked to Similac, EleCare, and Alimentum powdered infant formulas. Those infants are from Minnesota, Ohio, and Texas. The two infants who lived in Ohio died, although the FDA is investigating to see if Cronobacter sakazakii contributed to those deaths. Two recalls have been issued for those infant formula products. The first, on February 17, 2022, was for Similac, EleCare, and Alimentum powdered infant formula with … [Read more...]
Cronobacter Complications: Necrotizing Enterocolitis, Sepsis, and Meningitis
Cronobacter complications can include necrotizing enterocolitis (NEC), sepsis, and meningitis. These conditions can be fatal for infants, and a worry for parents in light of the Cronobacter illnesses linked to powdered infant formula. What are the symptoms of these conditions and what should parents look for? Four Infants Sick with Cronobacter First, some background. The Centers for Disease Control and Prevention (CDC) states that there are four infants from Minnesota, Texas, and Ohio who were sickened with Cronobacter infections after they were allegedly fed powdered infant formula produced by Abbott Nutrition at their Sturgis, Michigan plant. More reports of illness are being investigated. A recall has been issued for four types of that formula: Similac Pro Total Comfort, … [Read more...]
FDA Advice For Parents About Infant Formula Cronobacter Illnesses
This FDA advice for parents about infant formula and the Cronobacter illnesses linked to some types of powdered formula is offered to help parens navigate this difficult time. At least four infants, from Minnesota, Ohio, and Texas, are sick with Cronobacter sakazakii infections after consuming recalled Similac, EleCare, or Alimentum formula produced at Abbott Nutrition's Sturgis, Michigan plant. Because infant formula is the sole source of nutrition for many newborns and infants, the FDA is offering help. The FDA is stating that one infant allegedly contracted a Salmonella infection after being fed this formula, but the CDC is not including this illness in the case count until they complete their investigation. The key to the recall is the lot codes and expiration date on the … [Read more...]
CDC Weighs in on Cronobacter Illnesses and Infant Formula
The Centers for Disease Control and Prevention (CDC) is investigating Cronobacter illnesses and infant formula. Four infants who were allegedly fed powdered infant formula made by Abbott Nutrition at their Sturgis, Michigan facility have been diagnosed with Cronobacter sakazakii infections. The babies live in Minnesota (1), Ohio (2), and Texas (1). The two babies who lived in Ohio have sadly died. Cronobacter infections may have contributed to those deaths; the FDA and CDC are investigating. The infants allegedly consumed formula that included Similac Sensitive, Similac Pro-total Comfort, Similac Advance, and Similac PM 60/40. Abbott Nutrition issued a recall of certain lots of the first three types of formula on February 17, 2022, and added one lot of Similac PM 60/40 to the … [Read more...]
Similac PM 60/40 Infant Formula Recalled For Possible Cronobacter
One lot of Similac PM 60/40 Infant Formula (Lot number 27032K80 for the individual cans and Lot number 27032K800 for the case) has been voluntarily recalled by Abbott Nutrition after the company was informed of the death of an infant who allegedly consumed this product. The formula was manufactured in the company's Sturgis, Michigan plant. That baby tested positive for Cronobacter sakazakii. The case is under investigation since the cause of the infant's Cronobacter infection has not been determined. This product was not included in the original formula recall. No distributed product has tested positive for the presence of Cronobacter sakazakii, according to Abbott. And Abbott claims that recently tested product samples of Similar PM 60/40 infant formula with those lot numbers … [Read more...]