Live It Up Super Greens dietary supplement is being recalled for Salmonella contamination. All flavors of this product, including both Original and Wild Berry flavors with lots beginning with the letter "A" and all stick pack items are included in this recall. The recalling firm si Superfoods, Inc. doing business as Live It Up of New York, New York. There is a Salmonella outbreak linked to these products that has sickened at least 45 people in 21 states. Twelve people have been hospitalized because they are so sick. The recall notice states that, "At this time, the FDA and CDC have reported that the outbreak may be linked to Live it Up Super Greens," but both the FDA and CDC say that the product is linked to the illnesses. The case count by state is: Alabama (1), Connecticut (1), … [Read more...]
Sea Moss Gel Superfood Recalled For Possible Botulism
Sea Moss Gel Superfood in 14 flavors is being recalled for possible botulism contamination. The issue is a lack of required regulatory authorization and temperature monitoring records for pH-controlled food products. No illnesses have been reported to the company to date in connection with the consumption of these products. The recalling firm is Diva Farm of Los Angeles, California. PH-controlled food products that are not manufactured in accordance with regulatory requirements may present a potential risk of microbial growth, including organisms that can produce toxins associated with botulism. A tiny amount of botulism toxin, produced by spores from the bacteria Clostridium botulinum, can seriously injury or kill an adult. The recalled products include all sizes, flavors, and … [Read more...]
Moringa Leaf Powder Salmonella Outbreak Ends With 11 Sick
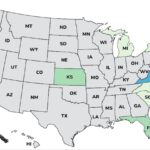
The Moringa leaf powder Salmonella outbreak has ended with 11 people sickened in seven states, according to the Centers For Disease Control and Prevention (CDC). Epidemiologic, traceback and laboratory data showed that products containing moringa leaf powder were contaminated with Salmonella Richmond and made people sick. The case count by state is: Florida (2), Kansas (2), Michigan (1), North Carolina (1), New York (1), South Carolina (1), and Virginia (3). Illness onset dates range from May 12, 2025 to September 4, 2025. The hospitalization rate is 27%, higher than the typical hospitalization rate for a Salmonella outbreak, which is 20%. The patient age range is from 13 to 65 years old. In interviews with public health officials, 9 out of 10 patients said they ate powdered … [Read more...]
Food to Live Moringa Powder Products Recalled For Salmonella
Food to Live Moringa Powder products are being recalled for Salmonella contamination. These items are linked to a nationwide Salmonella Richmond outbreak that has sickened at least 11 people in seven states and has hospitalized three patients. The two products are Moringa Leaf Powder and Organic Supergreens Powder Mix. The Moringa Leaf Powder was packaged in 8 ounce, 1 pound, 2 pound, 4 pound, 8 pound, 16 pound, and 44 pound bags. The Organic Supergreens Powder Mix was packaged in 8 ounce, 1 pound, 1.5 pound, 3 pound, 6 pound, and 12 pound bags. Only packages with lot codes starting with "SO-69006" and ending with "SO-72558" are included in this recall. These codes are printed on the back of the bag. No other Food to Live products and lots are included in this recall. The recall … [Read more...]
Two More Moringa Leaf Products Recalled With Links To Illnesses
Two more moringa leaf products have been recalled with links to illnesses in the Salmonella Richmond outbreak that has sickened at least 11 people in seven states, according to the FDA. The newly recalled products are Food to Live Organic Moringa Powder and Food to Live Organic Supergreens Powder Mix. Africa Imports Moringa Leaf Powder, previously recalled, had the "no illnesses linked to this product" statement removed. The case count by state is: Florida (2), Kansas (2), Michigan (1), North Carolina (1), New York (1), South Carolina (1), and Virginia (3). Illness onset dates range from May 12, 2025 to September 4, 2025. Three people have been hospitalized, for a hospitalization rate of 27%, which is higher than the typical 20% hospitalization rate for a Salmonella outbreak. The … [Read more...]
SiluetaYa Tejocote Root Recalled Because It is Yellow Oleander
SiluetaYa Tejocote Root is being recalled because the dietary supplement is actually yellow oleander, which is highly toxic. No illnesses have been reported to the company to date in connection with the consumption of this product. The recalling firm is SiluetaYa. Yellow oleander is a toxic plant that can cause serious illness and death. Yellow oleander can cause neurologic, gastrointestinal, and cardiovascular adverse health effects that can be severe or fatal. Symptoms may include nausea, vomiting, dizziness, diarrhea, abdominal pain, changes in heart rhythm, arrhythmia, and more. This product was sold online; the dates of sale were not provided in the recall notice. No UPC number was given. The recalled product is SiluetaYa Tejocote Root that is packaged in a round plastic 8 … [Read more...]
Monarch Kratom Recalled For Possible Salmonella Contamination
Monarch Kratom is being recalled for possible Salmonella contamination. No illnesses have been reported to the company to date in connection with the consumption of these products. The recalling firm is Vanguard Enterprises, doing businesss as DBA Bedrock Manufacturing of Boise, Idaho. These products were sold nationwide at the retail level except in the states of Alabama, Arkansas, Indiana, Rhode Island, and Wisconsin. They were also sold through mail order from the company website. They were sold between April 2023 to September 2023. The recalled products are all Monarch Premium Brand Kratom. They are packaged in 4 ounce mylar pouches. The products are packaged in multiple sizes. The product is in powder form. The recalled product sinlcude Bali Gold in 112 gram pouches with … [Read more...]
Organic Baby Bedtime Drops Recalled For Spoilage Concerns
Organic Baby Bedtime Drops are being recalled for possible spoilage concerns because of yeast contamination. Because this recall notice was posted on the FDA's Enforcement Reports Page, and not the regular recall page, there is no mention about whether or not any illnesses have been reported to the company to date in connection with the consumption of this item. The recalling firm is M.O.M. Enterprises of Richmond, California. This recalled product was sold at the retail level in these states: Alabama, Arkansas, Arizona, California, Colorado, Connecticut, Florida, Georgia, Iowa, Illinois, Indiana, Kansas, Kentucky, Michigan, Minnesota, Missouri, Mississippi, North Carolina, North Dakota, New Jersey, Nevada, New York, Ohio, Oregon, Pennsylvania, Rhode Island, South Carolina, … [Read more...]
Great Plains Bentonite + Herbal Detox Capsules Recalled
Great Plains Bentonite + Herbal Detox Capsules are being recalled for possible contamination with Pseudomonas aeruginosa. This pathogen can cause a range of symptoms in the GI tract including bloating, gas, diarrhea, and abdominal pain. Because this recall notice was posted on the FDA’s Enforcement Reports page, and not the regular recall page, there is no mention about whether or not any illnesses have been reported to the company in connection with the consumption of this product. The recalling firm is Yerba Prima Inc. of Ashland, Oregon. This product was sold at the retail level in these states: Arizona, California, Colorado, Connecticut, Florida, Georgia, Iowa, Illinois, Indiana, Kentucky, Maryland, Missouri, North Carolina, New Hampshire, Nevada, New York, Ohio, Pennsylvania, … [Read more...]