Live It Up Super Greens dietary supplement is being recalled for Salmonella contamination. All flavors of this product, including both Original and Wild Berry flavors with lots beginning with the letter "A" and all stick pack items are included in this recall. The recalling firm si Superfoods, Inc. doing business as Live It Up of New York, New York. There is a Salmonella outbreak linked to these products that has sickened at least 45 people in 21 states. Twelve people have been hospitalized because they are so sick. The recall notice states that, "At this time, the FDA and CDC have reported that the outbreak may be linked to Live it Up Super Greens," but both the FDA and CDC say that the product is linked to the illnesses. The case count by state is: Alabama (1), Connecticut (1), … [Read more...]
Lterfear Multifunction Pounding Game Recalled For Magnets
Lterfear Multifunction Pounding Game is being recalled because it contains high powered magnets that can detach, posing an ingestion hazard for children. When high powered magnets are swallowed, they can attract each other or other metal objects and become lodged in the digestive system. This can result in perforations, twisting or blockage of the intestines, blood poisoning, and death. No illnesses have been reported to the company to date. The retailer is Shenzhen Haichuan International Travel Service Co., Ltd., doing business as Findriver and Weeksome. This toy was manufactured in China. The recalled product is the Lterfear Multifunction Pounding Game. The model number is D888. The wooden game includes four games: whack-a-mole, fishing, xylophone, and carrot cutting. The toy … [Read more...]
Banana Pudding Ice Cream Recalled For Undeclared Soy
Banana Pudding Ice Cream is being recalled because it contains soy, one of the nine major food allergens, that is not declared on the product label as required by the FDA. Anyone who is allergic to soy could have a serious reaction if they eat this product. Because this recall notice was posted on the FDA's Enforcement Reports page, and not the regular recall page, there is no information about whether or not any allergic reactions have been reported to the company to date in connection with the consumption of this product. The recalling firm is House of Flavors of Ludington, Michigan. The recalled ice cream was sold at the retail level in these states: Alabama, Arizona, Connecticut, Georgia, Iowa, Illinois, Indiana, Kentucky, Michigan, Minnesota, Missouri, North Carolina, New … [Read more...]
Live It Up Super Greens Salmonella Outbreak Sickens 45
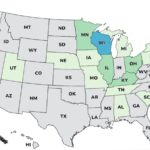
A Live It Up Super Greens Salmonella outbreak has sickened at least 45 people in 21 states, according to the Centers for Disease Control and Prevention (CDC). The company told the FDA today that they would initiate a voluntary recall, but that has not been announced. The case count by state is: Alabama (1), Connecticut (1), Delaware (1), Iowa (1), Maine (1), Michigan (1) Missouri (1), Nebraska (1), Pennsylvania (1), Tennessee (1), Utah (1), Washington (1), Massachusetts (1), New York (2), South Carolina (2), Vermont (2), Minnesota (3), Ohio (3), Illinois (4), Kentucky (4), and Wisconsin (11). Illness onset dates range from August 22, 2025 to December 30, 2025. The patient age range is from 16 to 81 years. Of the 41 people who gave information to public health officials, 12 have … [Read more...]
Outside the Breadbox Bread Crumbs Recalled For Egg and Milk
Outside the Breadbox Bread Crumbs are being recalled because they may contain eggs and milk, two of the nine major food allergens, that are not declared on the product label as required by the FDA. Anyone who is allergic to those ingredients, as well as anyone who is lactose intolerant, could have a serious reaction if they eat this product. No illnesses or allergic reactions have been reported to the company to date in connection with the consumption of this product. The recalling firm is VH Foods doing business as Outside the Breadbox of Colorado Springs, Colorado. This product was sold at the retail level in the state of Colorado and was also sold online. It was available for purchase between November 24, 2025 and December 1, 2025. The recalled product is Outside the Breadbox … [Read more...]
Sea Moss Gel Superfood Recalled For Possible Botulism
Sea Moss Gel Superfood in 14 flavors is being recalled for possible botulism contamination. The issue is a lack of required regulatory authorization and temperature monitoring records for pH-controlled food products. No illnesses have been reported to the company to date in connection with the consumption of these products. The recalling firm is Diva Farm of Los Angeles, California. PH-controlled food products that are not manufactured in accordance with regulatory requirements may present a potential risk of microbial growth, including organisms that can produce toxins associated with botulism. A tiny amount of botulism toxin, produced by spores from the bacteria Clostridium botulinum, can seriously injury or kill an adult. The recalled products include all sizes, flavors, and … [Read more...]
Arizona State Fair Source of 13 E. coli Illnesses in Children
According to information from the Maricopa County Department of Public Health, the Arizona State Fair is the source of 13 E. coli illnesses in children. Those children all had contact with petting zoos or other animal exhibits before they got sick. The state fair was held from September 19 to October 26, 2025. Shiga toxin-producing E. coli (STEC) bacteria can be shed by ruminant animals such as cows, goats, and sheep. Those animals do not have the vascular receptors for the Shiga toxins that STEC bacteria produce, so those toxins cannot destroy red blood cells in those animals as they do in people. Courtney Kreuzwiesner, Preparedness Communications Supervisor for the Maricopa County Department of Public Health told Food Poisoning Bulletin, "The Maricopa County Department of … [Read more...]
No Name Beef Burgers Recalled in Canada For E. coli O157:H7
No Name Beef Burgers are being recalled in Canada for possible E. coli O157:H7 contamination. No illnesses have been reported to the company to date in connection with the consumption of this product. The recalling firm is Loblaws Inc. These burgers were sold nationally at the retail level. The recalled product is no name Beef Burgers that are packaged in a 1.36 kilogram box. The box is yellow with a picture of the burger on the front and black printing. The UPC number that is stamped on the product label is 0 60383 37333 7. The best before date for the item is May 5, 2026, and the code is B13 BMP EST 112. If you purchased these burgers, do not eat them, even if you plan to cook them thoroughly to 160°F, because of the possibility of cross-contamination with other items and … [Read more...]
Mint Leaf Date Sweetened Chocolate Bar Recalled For Salmonella
Mint Leaf Date Sweetened Chocolate Bar is being recalled for possible Salmonella contamination. No illnesses have been reported to the company to date in connection with the consumption of this product. The recalling firm is Spring & Mulberry of Raleigh, North Carolina. This product was available for purchase online and through select retail locations nationwide since September 15, 2025. The recalled product is Mint Leaf Date Sweetened Chocolate Bar that weighs 2.1 ounces. The bar is wrapped in a teal paper wrapping with tan printing. The lot code, which is printed on the back of the packaging, is 025255. The company is proactively recalling this item. The potential for contamination was noted after routine testing was conducted by a third party lab. If you bought this … [Read more...]