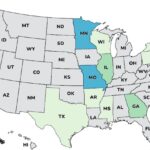
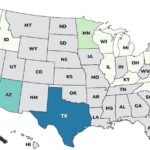
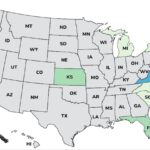
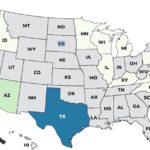
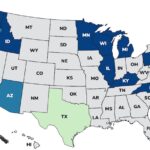
Continuing our countdown of the Top 10 Outbreaks of 2025, number 9 is the Metabolic Meals Salmonella outbreak that sickened at least 21 people in 13 states, according to the Centers For Disease Control and Prevention (CDC). The case count by state was: Arkansas (1), California (3), Connecticut (1), Georgia (2), Illinois (2), Minnesota (3), Missouri (3), Mississippi (1), New York (1), Pennsylvania (1), Texas (1), Washington (1), and Wisconsin (1). The patient age range is from 0 to 96 years. Illness onset dates range from July 24, 2025 to October 3, 2025. Of the 15 people who gave information to public health investigators, 13 said they ate a Metabolic Meals menu item before they got sick. Eight people have been hospitalized out of 19 who were interviewed, for a hospitalization … [Read more...]
Top 10 Outbreaks of 2024: Number 10 Sweet Cream Pastries
It's time for the Top 10 Outbreaks of 2025. Number 10 is the Sweet Cream Pastries Salmonella outbreak that sickened at least 18 people in seven states. Because the Centers for Disease Control and Prevention (CDC) did not release any information about this outbreak, we do not know the case count by state or the patient age range. The pastries were imported from Italy. The states where the patients lived were California, Illinois, Massachusetts, North Carolina, New Jersey, New York, and Pennsylvania. The last illness onset date was December 26, 2024. The outbreak was declared over in March 2025. Of the 12 patients who gave information about themselves to public health investigators, one person was hospitalized. Of seven people who responded, five, or 71%, said they ate pastries … [Read more...]
Vega Farms Eggs Salmonella Outbreak Sickens 63 in California
A Vega Farms eggs Salmonella Enteritidis outbreak has sickened at least 63 people in California, according to the California Department of Public Health (CDPH). Thirteen poeple have been hospitalized because they are so sick. Those numbers are current as of December 5, 2025. Public health officials are warning consumers not to eat, serve, or sell the recalled in-shell eggs. The eggs were recalled on December 5, 2025. The recalled product is Vega Farms Brown Eggs with handler code 2136. The Julian date (a 3-digit number from 001 to 365 that corresponds to the day of the year) is 328 and before. The sell by dates are 12-22-25 and earlier. The package sizes are 1 dozen cartons and 30 egg flats at the retail level, and 15 dozen cases that contain 6 flats of 30 eggs each at the … [Read more...]
Kanishka Cuisine of India Outbreak in Seattle Sickens 37
Kanishka Cuisine of India food poisoning outbreak in Seattle has sickened at least 37 people, according to the King County Health Department. That restaurant si s located at 1534 1st Avenue South in Seattle, Washington. The food was promoted as a Thanksgiving meal. The pathogen responsible for these illnesses has not been identified, and the outbreak is over. The event, "Fusion Thanksgiving Feast," consisted of food that was prepared at the restaurant for pick up by customers or delivery by the restaurant. Public health officials have not found a specific food or drink from the menu that caused this outbreak, but illness symptoms match those caused by bacterial toxins, such as those produced by Clostridium perfringens or Bacillus cereus. Those bacteria grow quickly in food when … [Read more...]
ByHeart Infant Botulism Outbreak Has Sickened 51 Babies
The ByHeart infant botulism outbreak has now sickened 51 babies in 19 states, according to the CDC. All of the patients have been hospitalized. That is an increase of 12 new cases, 12 new hospitalizations, and one new state since the last update was issued a week ago. The new state is Ohio. The case count by state is: Arizona (5), California (12), Idaho (2), Illinois (2), Kentucky (1), Massachusetts (2), Maine (1), Michigan (1) Minnesota (3), North Carolina (2), New Jersey (1), Ohio (1), Oregon (4), Pennsylvania (1), Rhode Island (1), Texas (8), Virginia (1), Washington (2), and Wisconsin (1). The infants range in age from 16 to 264 days. Illness onset dates range from December 24, 2023 to December 1, 2025. All of the infants were hospitalized and treated with BabyBIG®, the … [Read more...]
Wild Mushrooms Sicken 21 in California With Amatoxin Poisoning
Wild mushrooms have sickened 21 people in California with amatoxin poisoning, according to the California Department of Public Health (CDPH). Those sickened have severe liver damage, and one adult died. The patients include adults and children. Significant clusters are in the San Francisco Bay and Monterey areas, although the risk is statewide. Dr. Erica Pan, CDPH Director and State Public Health Officer, said in a statement, "Death cap mushrooms contain potentially deadly toxins that can lead to liver failure. Because the death cap can easily be mistaken for edible safe mushrooms, we advise the public not to forage for wild mushrooms at all during this high-risk season." Mushroom identification can be tricky, and even experts can be fooled. Many toxic mushrooms such as the death … [Read more...]
Increase in Campylobacter and STEC in Idaho in Raw Milk Drinkers
There is an increase in Campylobacter and STEC infections linked to raw milk in Idaho, according to the Idaho Department of Health and Welfare. Since August 1, 2025, at least 23 cases of Campylobacter infections, including six children under the age of 12, along with three cases of Shiga toxin-producing E. coli (STEC) infections, have been reported among raw milk consumers in that state. No raw milk producer, dairy, or grocery store was named in the outbreak notice. All the notice said was that, "People should be aware of possible health risks before consuming raw, unpasteurized dairy products or providing such products to family members, particularly people who might be at higher risk for illness, including young children, pregnant women, the elderly, and those who are … [Read more...]
Moringa Leaf Powder Salmonella Outbreak Ends With 11 Sick
The Moringa leaf powder Salmonella outbreak has ended with 11 people sickened in seven states, according to the Centers For Disease Control and Prevention (CDC). Epidemiologic, traceback and laboratory data showed that products containing moringa leaf powder were contaminated with Salmonella Richmond and made people sick. The case count by state is: Florida (2), Kansas (2), Michigan (1), North Carolina (1), New York (1), South Carolina (1), and Virginia (3). Illness onset dates range from May 12, 2025 to September 4, 2025. The hospitalization rate is 27%, higher than the typical hospitalization rate for a Salmonella outbreak, which is 20%. The patient age range is from 13 to 65 years old. In interviews with public health officials, 9 out of 10 patients said they ate powdered … [Read more...]
ByHeart Formula Botulism Outbreak Sickens Two More Infants
The ByHeart formula botulism outbreak has sickened two more infants, bringing the total of sick babies to 39, according to the CDC. That is an increase from 37 sick since the last update was issued on November 26, 2025. And one more state is included: Virginia. The case count by state is: Arizona (3), California (5), Idaho (1), Illinois (2), Kentucky (1), Massachusetts (2), Maine (1), Michigan (1) Minnesota (2), North Carolina (2), New Jersey (1), Oregon (4), Pennsylvania (1), Rhode Island (1), Texas (8), Virginia (1), Washington (2), and Wisconsin (1). For 38 infants whose age was made available to public health officials, they range in age from 16 to 264 days. Illness onset dates range from August 9 to November 19, 2025 for 38 of these infants. All of the infants were hospitalized … [Read more...]
ByHeart Formula Botulism Outbreak Case Count Up to 37 Infants
The ByHeart formula botulism outbreak case count has now sickened 37 infants in 17 states, according to the Centers for Disease Control and Prevention (CDC). That's an increase of six new patients, six new hospitalizations, and two new states since the last update was issued on November 20, 2025. The new states are Massachusetts and Wisconsin. The case count by state is: Arizona (3), California (5), Idaho (1), Illinois (2), Kentucky (1), Massachusetts (1), Maine (1), Michigan (1) Minnesota (2), North Carolina (2), New Jersey (1), Oregon (3), Pennsylvania (1), Rhode Island (1), Texas (8), Washington (2), and Wisconsin (1). For 35 infants whose age was made available to public health officials, they range in age from 16 to 264 days. Illness onset dates range from August 9 to November … [Read more...]