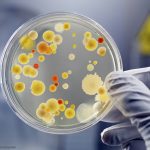
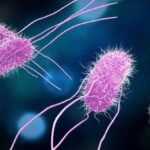
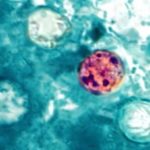
A leafy greens Salmonella outbreak announcement was just released by the FDA in a heavily redacted report. Forty people were sickened with Salmonella Lomalinda infections; five of those patients were hospitalized. The FDA was notified of the outbreak by the Centers for Disease Control and Prevention (CDC) on September 9, 2025, stating that seven people were sick. On September 11, 2025, the CDC named leafy greens as the leading "vehicle of interest" in the outbreak. The FDA initiated a traceback investigation consisting of redacted legs representing the patients, who said they consumed ready to eat organic baby spring mix salads before getting sick. The establishments that sold the greens were redacted, as were the states where the patients live. The salads were … [Read more...]
FDA Seizes 7-OH Opioids in Missouri to Protect Consumers
The FDA has seized 7-OH opioids in Missouri to protect consumers. The U.S. Marshals Service took about 73,000 units of 7-hydroxymitragynine (7-OH) products that are valued at roughly $1 million from three firms in that state. The firms were not named. The seizure included foods and dietary supplement products, including liquid shots and tablets, that contain concentrated 7-OH as an ingredient. This ingredient is recognized as having potential for abuse because it binds to opioid receptors. It can't be legally added to supplements or conventional foods. The FDA has not approved 7-OH for medical use and it does not meet safety standards. The FDA worked with the Missouri Department of Health and Senior services in this enforcement action. In July 2025, the FDA recommended the … [Read more...]
FDA Releases Two New Tools in Foodborne Illness Investigations
The FDA has released two new tools in their foodborne illness investigations: EIS (Executive Incident Summary Abstracts) and FOOD (Foodborne Outbreak Overview of Data). The EIS will be released after an outbreak is over, providing high level information about the outbreak and any new information that may have been discovered since the investigation ended. FOOD is a comprehensive detailed report on product-pathogen pairs that have caused repeated outbreaks over the years. The EIS reports are written after the investigation into an FDA-regulated human food product is closed. The reports will be redacted to protect confidential information as well as personally identifiable information and other info that the Freedom of Information Act (FOIA) states is exempt from disclosure to the … [Read more...]
FDA Advises Consumers Not to Eat Indonesian Imported Shrimp
The FDA is advising consumers to not eat, sell, or serve certain Indonesian imported shrimp imported from PT Bahari Makmur Sejati. The shrimp is in violation of the Federal FD&C Act because it may be contaminated with cesium-137 and may be radioactive. The problem is that shipping containers have tested positive for the radioactive isotope. No radioactive shrimp has entered U.S. commerce. This list will be updated as the FDA updates. Three firms have issued press releases for recalled products. They are: August 21, 2025: Southwind Foods, LLC Recall August 22, 2025: Beaver Street Fisheries, LLC Recall August 27, 2025: AquaStar (USA) Corp Recall – Kroger Brand August 28, 2025: AquaStar (USA) Corp Recall – Aqua Star Brand August 29, 2025: Southwind Foods, LLC Recall … [Read more...]
Nicotine Pouch Makers Asked to Use Child Resistant Packaging
Nicotine pouch makers are being asked by the FDA to use child resistant packaging. The number of nicotine exposure cases reported to U.S. Poison Control Centers have steadily increased from April 1, 2022 to March 31, 2025, according to public health officials. These pouches contain concentrated nicotine that can be harmful or even fatal to young children, even when consumed in small amounts. Doses as low as 1 to 4 milligrams have caused toxic effects in young children. Symptoms of nicotine poisoning can include vomiting, confusion, and loss of consciousness. FDA Commissioner Marty Makary, M.D., M.P.H. said in a statement, "I am concerned about rising reports of nicotine exposures in young children caused by nicotine pouches. The fruity flavors and bright, colorful designs of … [Read more...]
FDA Warns About Products From East Africa Boutique
The FDA is warning consumers, retailers, and distributors about products from Pan-African Food Distributors doing business as East Africa Boutique because they were held under insanitary conditions and could be contaminated with filth. These items include infant nutritional cereals, baking ingredients, and other food products. The products may or may not have a label with the firm name. The warning was updated on June 3, 2025 to include cosmetics. Products that are held under insanitary conditions could pose a serious health risk and could lead to serious illness, including leptospirosis, hantavirus infection, salmonellosis, yersiniosis, E. coli infections, and rat-bite fever. You can see the long list of products, along with package sizes, lot codes, and expiration dates at the … [Read more...]