The FDA has issued final guidance for lead in children's food. These action levels reflect the levels of lead at which the FDA may regard the food as adulterated. While there are no safe levels of lead consumption, it is not possible to completely remove lead from every food as the heavy metal occurs naturally and is found in air, water, and soil. This guidance supports the Closer to Zero initiative to reduce exposure to contaminants through food to as low as possible. The action levels are 10 parts per billion for fruits, vegetables (except single ingredient root vegetables), mixtures (including grain and meat based mixtures), yogurt, custards and puddings, and single ingredient meats. The level is 20 parts per billion for single ingredient root vegetables, and 20 parts per billion … [Read more...]
FDA Issues Final Compliance for Preventing Scombrotoxin in Fish
The FDA has issued a final compliance policy guide (CPG) for preventing Scombrotoxin in fish. The adulteration of fish and fishery products with histamines can cause Scombrotoxin poisoning in humans. Histamine production can occur in fish as the flesh begins to decompose after death. The CPG will help the FDA address adulteration during surveillance sampling and testing and will increase consumer protection by lowering the levels of histamine in fish. In some species of fish, such as tuna and mahi-mahi, the muscle tissue can cause histamines to form because of the activity of enzymes produced by spoilage bacteria. Unless fish are quickly and properly chilled after the catch, histamines can accumulate. Once they are formed, these histamines cannot be removed by washing, freezing, … [Read more...]
FDA Finds More Supplements That Contain Yellow Oleander
The FDA has found more supplements that contain yellow oleander, a toxic plant that can cause serious illness and death. Yellow oleander can cause neurologic, gastrointestinal, and cardiovascular adverse health effects that can be severe or fatal. These three new products should not be consumed. If you have purchased them, take them back to the place of purchase or discard them according to your community's methods of getting rid of toxic substances. You can see pictures of these items at the FDA web site. The products include Creative Interiors, LLC/Privit Wellness, LLC Primor Health Optimus Weight. It was sold through Amazon. The firm has not yet committed to a recall. The second product is Innovacion Natural LLC SdB Elite Salud de Belleza that was also sold on Amazon. The FDA … [Read more...]
FDA Has Started a Streamlined Food Complaints Program
Have you ever wanted to tell the FDA about a problem with food? The FDA has started a streamlined food complaints program to make the process easier. The complaints can be about an illness, an injury, an allergic reaction, concerns about a dietary supplement, or any concern about defects in the safety or quality of an FDA-regulated food. This is part of the Human Foods Program. Until this new program was initiated, consumer complaints were directed to Consumer Complaint Coordinators in the FDA's Office of Regulatory Affairs. The phone numbers associated with those coordinators will no longer be active. Instead, consumers can call 1-888-SAFEFOOD (1-800-723-3663). Then, officials with the Human Foods Program will receive, triage, and evaluate complaints, and the new Office of … [Read more...]
FDA Revokes Regulation Allowing Brominated Vegetable Oil
The FDA has revoked the regulation allowing brominated vegetable oil (BVO) in food. This oil is modified with bromine. He agency concluded that the intended use of BVO in food is no longer considered safe. A series of studies conducted in collaboration with the National Institutes of Health found the potential for adverse human health effects from the use of brominated vegetable oil. The FDA announced plans to ban this product last year. And the state of California banned BVO along with other compounds before the FDA announced their decision. Brominated vegetable oil was removed from the Generally Regarded as Safe (GRAS) list in 1970. It was used in small amounts to keep the citrus flavoring from floating to the top in some beverages. Few beverages in the United States contain … [Read more...]
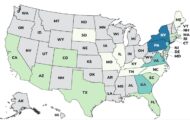
Do Not Eat Certain Shellfish From OR and WA Because of PSP
The FDA says consumers should not eat certain shellfish harvested from Oregon and Washington waters because they may be contaminated with paralytic shellfish poison (PSP). Restaurants and retailers should not sell these products. Retailers and restaurants in Arizona, California, Colorado, Hawaii, Nevada, New York, Oregon, and Washington may have purchased oysters and bay clams from growing areas in Netarts Bay and Tillamook bay, Oregon harvested on or after May 28, 2024. All shellfish from growing areas in Willapa Bay, Washington Stony Point, harvested between May 26, 2024 and May 30, 2024;5/26/24; from Bay Center, harvested between May 29, 2024 and May 30, 2024; and Bruceport, harvested between May 29, 2024 and May 30, 2024 are included. Consumers in Arizona, California, … [Read more...]
New Salmonella Outbreak on FDA Core Table Sickens 100
A new Salmonella outbreak on the FDA's Core Outbreak Investigation Table has sickened at least 100 people, according to the latest update. There are only two other outbreaks on the Table at this time: The E. coli O157:H7 outbreak allegedly linked to walnuts, and the Salmonella Typhimurium outbreak that is allegedly linked to organic fresh basil. The new Salmonella outbreak is caused by Salmonella Africana, a variant that has not caused any illnesses in the United States since at least 2011. There are 100 people sick. The FDA has initiated traceback, but has not started inspections, and has not collected or tested samples. The E. coli O157:H7 outbreak allegedly linked to organic Gibson Farms walnuts remains unchanged since the last update was issued on May 1, 2024. There are 12 … [Read more...]