The Aladdin Renaissance Cafe Salmonella outbreak in San Diego County in California has sickened at least 98 people. This outbreak was first announced by the San Diego County Health Department on May 2, 2025. Those 98 cases are both confirmed and probable. At least 10 people have been hospitalized. The restaurant was closed on May 2 and reopened on May 13, 2025 when the health department said it was safe to serve food again. The patient age range is apparently from age one to 90. Patients ate at the restaurant between April 25 and May 1, 2025. We still don't know what caused the outbreak. As in all restaurant outbreaks, it could have been a sick employee who handled food improperly, or it could have been contaminated food. The Health Department has not announced a … [Read more...]
New Bedner Cucumber Salmonella Outbreak Sickens 26
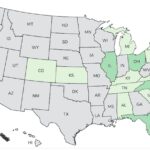
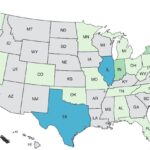
A new Bedner cucumber Salmonella outbreak has sickened at least 26 people in 15 states. Last year the number one outbreak was a Salmonella outbreak linked to that brand of cucumbers, sickening at least 551 people and hospitalizing 155. The case count by state is: Alabama (1), California (1), Colorado (1), Florida (3), Illinois (4), Kansas (1), Kentucky (1), Michigan (1), North Carolina (3), New York (1), Ohio (4), Pennsylvania (2), South Carolina (1), Tennessee (1), and Virginia (1). The patient age range is from 2 to 69 years, and illness onset dates range from April 2, 2025 to April 28, 2025. Nine people have been hospitalized. Seven of the patients reported taking a cruise before they got sick. All of the cruises departed from Florida. Those patients were on cruise … [Read more...]
Aladdin Mediterranean Cafe Salmonella Outbreak Sickens 37
The Aladdin Mediterranean Cafe Salmonella outbreak has now sickened at least 37 people, according to an update posted by San Diego County Public Health Services. Nine people have been hospitalized becasue they are so sick. That's an increase of 23 more patients and four more hospitalizations since the original notice was posted on May 1, 2025. The restaurant is located at 5420 Clairemont Mesa Boulevard in San Diego. Management voluntarily closed the restaurant on May 1, 2025. They have been working closely with the county's Environmental Health and Quality Department along with Public Health Services on the investigation. The patient age range is from 1 to 90 years old. Patients ate at the restaurant between April 25 and May 1, 2025. Illness onset dates were not reported in … [Read more...]
Sushi Train Restaurant Allegedly Site of Food Poisoning Outbreak
Sushi Train restaurant in Nashville is allegedly the site of a food poisoning outbreak, according to news reports. The Metro Public Health Department is investigating. Around 12 customers reported "rapid-onset" food poisoning after eating at the restaurant, which is located at 94 White Bridge Road in Nashville. The patients reported severe food poisoning symptoms. Illness onset dates appear to be late March through late April 2025, according to reports on I Was Poisoned. The Tennessean obtained a report on these illnesses from the health department. The types of symptoms were not mentioned, so it's difficult to speculate about which pathogen may have caused these illnesses. Rapid onset does suggest norovirus, Staphylococcus aureus, or Bacillus cereus. That restaurant has … [Read more...]