Fiji Natural Artesian Water is being recalled because it contains manganese and three bacterial genera. Because this recall notice was posted on the FDA's Enforcement Reports page and not the regular recall page, there is no information about whether or not any adverse events have been reported to the company to date in connection with the consumption of this product. The recalling firm is Natural Waters of Viti Limited of Tavua, Fiji. Water with high levels of manganese can cause adverse events in some people. This product should not cause serious health events. The recalled product is Fiji Natural Artesian Water that is in a 500 milliliter bottles that are packaged in a 24 pack container. The case UPC number on the label is 6 32565 00004 3, and the individual bottle UPC number … [Read more...]
FDA Warns About Toxic Amygdalin in Apricot Seeds
The FDA is warning consumers about toxic amygdalin in apricot seeds that can lead to cyanide poisoning. The FDA reviewed analytical packets from the Commonwealth of Virginia Division of Consolidated Laboratory Services (DCLS) from three samples collected by the Texas Department of State Health Services (DSHS), All of them contained the toxic compound amygdalin. When consumed, amygdalin can lead to fatal cyanide toxicity. Mild to moderate symptoms of this toxicity include difficulty breathing, bluish discoloration of the skin or mucous membrane, weakness, and lightheadness. Symptoms of severe acute toxicity include coma, seizures, stupor, dysrhythmias, cardiovascular collapse, and metabolic acidosis. Chronic consumption of foods that have high concentrations of cyanogenic glycosides … [Read more...]
Types of Rizo-Lopez Foods Recalled For Possible Listeria
The Centers for Disease Control and Prevention (CDC) has published an overall list of the types of Rizo-Lopez foods that have been recalled in the wake of a deadly Listeria monocytogenes outbreak. Both FDA and USDA regulated foods are included on this list. The outbreak has sickened at least 26 people in 11 states. Twenty-three of the patients have been hospitalized, and two people have died. The case count by state remains at: Arizona (4), California (8), Colorado (4), Florida (1), Georgia (1), Nevada (1), North Carolina (1), Oregon (1), Tennessee (2), Texas (2), and Washington (1). The patient age range is from less than one to 88 years. Illness onset dates range from June 15, 2014 to December 10, 2023. The patients who died lived in Texas and California. All Rizo-Lopez dairy … [Read more...]
Monster Green Energy Drink Recalled For Foreign Material
Monster Energy brand Monster Green Energy Drink is being recalled in Canada for possible foreign material contamination. The product may contain pieces of film from delamitation of the can coating. This can pose a choking hazard. The recall notice did not state whether or not any adverse reactions have been reported to the company to date in connection with the consumption of this product. The recalling firm is Monster Energy Canada Ltd. The product was sold at the retail level nationally. The recalled product is Monster Energy brand Monster Green Energy Drink that is packaged in 473 milliliter containers. The codes for this recalled product, which are the expiration dates, that are stamped on the can are SE2225RD, SE1525RD, and OC1325RD. The UPC number for the item that is printed … [Read more...]
Deadly Cantaloupe Salmonella Outbreak Ends in Canada and US
The deadly cantaloupe Salmonella outbreak has ended in Canada and the United States, with almost 600 sick, 226 hospitalized, and 15 deaths. The Centers for Disease Control and Prevention declared the U.S. outbreak over on January 19, 2024, and Public Health Canada declared it over on January 29, 2024. The case count by state in the U.S. is: Alaska (1), Arizona (15), Arkansas (2), California (56), Colorado (11), Connecticut (2), Florida (4), Georgia (8), Illinois (22), Indiana (9), Iowa (12), Kansas (2), Kentucky (10), Maryland (9), Massachusetts (2), Michigan (7), Minnesota (29), Mississippi (1), Missouri (15), Montana (3), Nebraska (7), Nevada (5), New Hampshire (1), New Jersey (8), New Mexico (2), New York (14), North Carolina (7), North Dakota (1), Ohio (14), Oklahoma (4), … [Read more...]
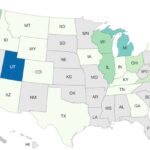
Number Two Outbreak of 2023: Deadly Gills Onions Salmonella
The number two outbreak of 2023 is the deadly Gills Onions Salmonella Thompson outbreak that sickened at least 80 people in 23 states. Eighteen people were hospitalized, and one person who lived in Wisconsin died. The outbreak was declared over in mid-December 2023. The case count by state was: Arizona (1), California (17), Colorado (1), Georgia (1), Iowa (1), Idaho (1), Illinois (4), Indiana (2), Kentucky (1) Massachusetts (1), Michigan (6), Montana (2), North Dakota (2), New York (1), Ohio (3), Oregon (3), Tennessee (1), Texas (1), Utah (19), Virginia (3), Washington (4), Wisconsin (4), and Wyoming (1). Illness onset dates ranged from August 2, 2023 to November 11, 2023. The patient age range was from less than one to 90 years. The government interviewed patients about the … [Read more...]
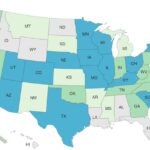
Deadly Gills Onions Salmonella Outbreak Ends With 80 Sick
The deadly Gills Onions Salmonella outbreak has ended with 80 people sick in 23 states, according to the Centers for Disease Control and Prevention (CDC). Eighteen people have been hospitalized. That is an increase of seven more patients and one more state since the last update was announced on October 24, 2023. One person who lived in Wisconsin has sadly died. The case count by state is: Arizona (1), California (17), Colorado (1), Georgia (1), Iowa (1), Idaho (1), Illinois (4), Indiana (2), Kentucky (1) Massachusetts (1), Michigan (6), Montana (2), North Dakota (2), New York (1), Ohio (3), Oregon (3), Tennessee (1), Texas (1), Utah (19), Virginia (3), Washington (4), Wisconsin (4), and Wyoming (1). Illness onset dates range from August 2, 2023 to November 11, 2023. The patient age … [Read more...]
Cantaloupe Salmonella Cases Almost Double to 230 Sick
Cantaloupe Salmonella cases have almost doubled, according to the CDC, to 230 sick, an increase of 113 new cases since the last update was issued on November 30, 2023. One new death has been reported, 96 people are hospitalized, and there are four new states in the case count total. The case count by state is: Alaska (1), Arkansas (1), Arizona (15), California (8), Colorado (8), Georgia (3), Iowa (5), Illinois (11), Indiana (5), Kansas (2), Kentucky (7), Massachusetts (1), Maryland (2), Michigan (2), Minnesota (20), Missouri (14), Mississippi (1), Montana (2), North Carolina (4), Nebraska (7), New Jersey (3), New Mexico (2), Nevada (5), New York (7), Ohio (8), Oklahoma (3), Oregon (5), Pennsylvania (5), Rhode Island (1), South Carolina (6), South Dakota (1), Tennessee (5), Texas … [Read more...]
Deadly Cantaloupe Salmonella Outbreak Sickens 162 in US, Canada
The deadly cantaloupe Salmonella outbreak has sickened at least 162 people in the United States and Canada. Three people have died; two in Minnesota in the U.S. and one in Canada. And 62 are in the hospital; 45 in the United States and 17 in Canada. In the U.S., the case count by state is: Arkansas (1), Arizona (7), California (1), Colorado (2), Georgia (3), Iowa (5), Illinois (4), Indiana (2), Kentucky (5), Massachusetts (1), Maryland (1), Michigan (1), Minnesota (13), Missouri (9), Mississippi (1), North Carolina (2), Nebraska (4), New Jersey (1), Nevada (2), New York (1), Ohio (8), Oklahoma (1), Oregon (1), Pennsylvania (1), Rhode Island (1), South Carolina (3),Tennessee (4), Texas (3), Utah (1), Virginia (1), Washington (1), and Wisconsin (8). In Canada, the case count by … [Read more...]